Design of Phase II Clinical Trials - UC Davis Health
Drug is a vascular endothelial growth factor (VEGF) inhibitor 2 dose levels tested (2 mg/kg and 4 mg/kg), based on previous phase 1 & 2 studies Patients with advanced platinum-resistant ovarian cancer Simon minimax 2-stage design Primary outcome: objective response rate (ORR) Null hypothesis: ORR ≤ 5%
Download Design of Phase II Clinical Trials - UC Davis Health
Information
Domain:
Source:
Link to this page:
Please notify us if you found a problem with this document:
Documents from same domain
MANDATORY ANNUAL TRAINING MANUAL
health.ucdavis.eduUC Davis Health Mandatory Annual Training Manual 2021 1 Version: January 2021 - FINAL MANDATORY ANNUAL TRAINING MANUAL 2021
Training, Manual, Annual, Mandatory, Mandatory annual training manual
Helpful Guidelines for Successful Weight Loss
health.ucdavis.eduUse low-fat cooking methods such as baking, grilling, boiling, poaching, broiling, roasting, steaming, or microwaving without adding fat. Avoid frying. Place meat on a rack so the fat will drain off during cooking. Trim all visible fat and skin from poultry and meat before cooking. Use nonstick cookware or cooking sprays.
Diversity, Equity, and Inclusion Curriculum
health.ucdavis.eduDefine structural competency and structural violence ... microagressions- triangle model framework Apply ARISE Practice modeling bystander interventions (with u se of role play) in clinical scenarios Addressing Microaggressions in Academic Health- utilize triangle model (Ackerman Barger) ...
Healthy Eating During Pregnancy - UC Davis Health
health.ucdavis.eduHEALTHY EATING DURING PREGNANCY . During pregnancy you will need to meet the nutrition needs of both . you and your baby through the foods you choose to eat and drink. • During the first trimester (weeks 1 – 12) your calorie needs do not change. For good nutrition, choose a variety of foods including: fruits, vegetables, low-fat dairy,
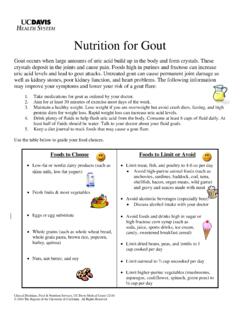
Nutrition for Gout
health.ucdavis.eduuric acid levels and lead to gout attacks. Untreated gout can cause permanent joint damage as well as kidney stones, poor kidney function, and heart problems. The following information may improve your symptoms and lower your risk of a gout flare: 1. Take medications for gout as ordered by your doctor.
INTRAVENOUS VANCOMYCIN DOSING AND MONITORING …
health.ucdavis.eduADULT INTRAVENOUS VANCOMYCIN DOSING AND MONITORING GUIDELINES DOSE: Adult dose: (based on actual body weight (ABW))*,^: 12.5 to 15 mg/kg (round off to nearest 250 mg increment, to max dose of 1500mg; see dosing table) * If ABW is > 30% ideal body weight (IBW), then use adjusted body weight = IBW + 0.4(Total body weight - IBW) IBW Males = 50 …
Pain Self-Management Strategies - UC Davis Health
health.ucdavis.eduImagery typically works best with eyes closed and muscles relaxed. The place you imagine can be one that you have actually visited or one you develop by just ... For example, imagine what kind of day it is, the look of the sky, and all the things you see around you. Imagine the beautiful colors you see, enjoyable scents you notice, and the ...
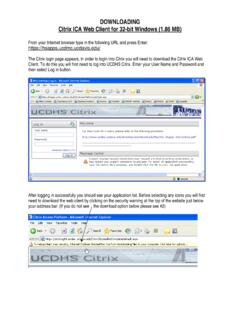
DOWNLOADING Citrix ICA Web Client for 32-bit Windows …
health.ucdavis.eduCitrix ICA Web Client for 32-bit Windows (1.86 MB) From your Internet browser type in the following URL and press Enter: https://hsapps.ucdmc.ucdavis.edu/ The Citrix login page appears, in order to login into Citrix you will need to download the Citrix ICA Web Client. To do this you will first need to log into UCDHS Citrix.
Allergic Cross-reactivity of Select Antimicrobials
health.ucdavis.eduType I hypersensitivity reactions are IgE-mediated responses that manifest clinically as urticaria, angioedema, anaphylaxis, or anaphylactic shock and are potentially fatal. These are true hypersensitivity reactions caused by specific antibodies to drugs. Onset is usually within 30-60 minutes of drug administration.
Challenges of Retrospective studies 8March2017 Kim
health.ucdavis.eduBecause retrospective observational studies use data that were originally collected for other purposes, not all the relevant information may have been available for analysis. There are also unknown potential confounders. We need methods to improve the comparability of the intervention and control groups. The methods include:
Related documents
Common Terminology Criteria for Adverse Events (CTCAE)
ctep.cancer.govCommon Terminology Criteria for Adverse Events (CTCAE) Version 5.0 . Published: November 27, 2017 . U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES . National Institutes of Health
Events, Criteria, Terminology, Common, Adverse, Common terminology criteria for adverse events
Classification and Pathology of Lung Cancer
cbc.org.brvascular endothelial growth factor inhibitor bevacizumab in patients with SCC can potentially precipitate life-threatening pulmonary hemorrhage, and therefore, should be avoided.28,29;. lung cancer. Classification and Pathology of Lung Cancer
Syndrome mRN A Vaccines Put You at Risk for Acute Coronar y
media.mercola.comEndothelial Inammator y Markers and ACS Risk as Measured by the PULS Cardiac Test: ... Hepatocyte growth factor (HGF), a marker for chemotaxis of T-cells into epithelium ... you basically end up with so many blood clots throughout your vascular. system that your coagulation system is exhausted, hence the low platelet count. The.
ISSVA classification for vascular anomalies
www.issva.orgof vascular malformations and tumors continues to grow Overview table °defined as two or more vascular malformations found in one lesion * high-flow lesions A list of causal genes and related vascular anomalies is available in Appendix 2 The tumor or malformation nature or precise classification of some lesions is still unclear. These lesions
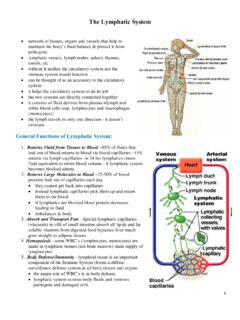
The Lymphatic System
www.soinc.org• Fluid that leaks from the vascular system is returned to general circulation via lymphatic vessels. • Lymph vessels act as a reservoir for plasma and other substances including cells that leaked from the vascular system • The lymphatic system provides a one-way route for movement of interstitial fluid to the cardiovascular system.
WORLD ANTI-DOPING CODE PROHIBITED LIST
www.wada-ama.org• Vascular endothelial growth factor (VEGF) and other growth factors or growth factor modulators affecting muscle, tendon or ligament protein synthesis/degradation, vascularisation, energy utilization, regenerative capacity or fibre type switching. 3. GROWTH FACTORS AND GROWTH FACTOR MODULATORS S2 PEPTIDE HORMONES, GROWTH FACTORS, …
Lists, Growth, Prohibited, Vascular, Vascular endothelial growth, Endothelial, Prohibited list
WORLD ANTI-DOPING CODE INTERNATIONAL STANDARD …
www.wada-ama.org• Vascular endothelial growth factor (VEGF) and other growth factors or growth factor modulators affecting muscle, tendon or ligament protein synthesis/degradation, vascularisation, energy utilization, regenerative capacity or fibre type switching. 3. GROWTH FACTORS AND GROWTH FACTOR MODULATORS S2 PEPTIDE HORMONES, GROWTH FACTORS, …
WOUND EXUDATE - WUWHS
www.wuwhs.orgGrowth factors Lower Growth factors stimulate the proliferation and migration of cells involved in new blood vessel formation, epithelialisation, wound contraction and the deposition of extracellular matrix. In non-healing wounds, levels of growth factors are lower than in healing wounds, probably mainly because of degradation by proteolytic ...