19 Monoclonal
Found 5 free book(s)Talking with Patients about Monoclonal Antibodies for ...
combatcovid.hhs.govfor COVID-19, early treatment with monoclonal antibodies may prevent progressing to more severe disease and hospitalization. Q: Why am I eligible for the treatment? A: Monoclonal antibody treatments may help people who: • Have mild to moderate symptoms of COVID-19, and • Have tested positive for COVID-19, and • Have had symptoms for 10 ...
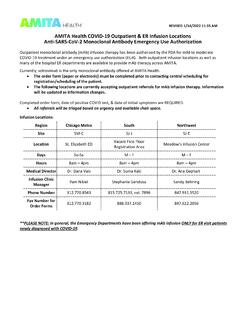
AMITA Health COVID-19 Outpatient & ER Infusion Locations ...
www.amitahealth.orgAMITA Health COVID-19 Outpatient & ER Infusion Locations . Anti-SARS-CoV-2 Monoclonal Antibody Emergency Use Authorization . Outpatient monoclonal antibody (mAb) infusion therapy has been authorized by the FDA for mild to moderate COVID-19 treatment under an emergency use authorization (EUA). Both outpatient infusion locations as well as
YOU HAVE COVID-19 INFECTION, NOW WHAT?
www.health.nd.govJan 12, 2021 · You should wait until your isolation is over before receiving the COVID-19 vaccine; if you received monoclonal antibody therapy for COVID-19, you should wait 90 days before receiving the COVID-19 vaccine. YOU HAVE COVID-19 INFECTION, NOW WHAT? Document last reviewed: 1/12/2021
PENNSYLVANIA DEPARTMENT OF HEALTH PAHAN 8-19-UPD …
www.health.pa.govAug 19, 2021 · The FDA issued EUAs for certain anti-SARS-CoV-2 monoclonal antibodies, including combination therapy casirivimab plus imdevimab, for treatment of COVID-19 infection. The EUAs for treatment of COVID-19 allow for use of the agents in nonhospitalized patients age 12 or older and weighing 40kg
Monoclonal Antibody Therapy for COVID-19
www.bop.govFederal Bureau of Prisons Monoclonal Antibody Therapy for COVID-19 Clinical Guidance December 2020 . 3 . C. LINICAL . P. RESENTATION. Patients with risk factors for severe COVID-19 illness and one or more of the following mild or moderate COVID - 19 symptoms . may be considered for treatment. with mAbs for COVID-19: • Fever • Cough • Sore ...