Conductivity Of Electrolytes In Solution
Found 9 free book(s)Electrical Conductivity of Aqueous Solutions
www.colby.edurelationship between conductivity and strong and weak electrolytes. To do this, you will add increasing amounts of either acid or base to several electrolyte solutions. After each addition you will measure the conductivity of the solution. From the conductivity data, you will work to deduce the nature of the
THEORY AND APPLICATION OF CONDUCTIVITY
www.emerson.comConductivity is a measure of how well a solution conducts electricity. To carry a current a solution must contain charged particles, or ions. Most conductivity measurements are made in aqueous solutions, and the ions responsible for the conductivity come from electrolytes dissolved in the water. Salts (like sodium
Chemistry Notes for class 12 Chapter 3 Electrochemistry
ncerthelp.com(i) Nature of electrolyte The strong electrolytes like KNO 3 KCl. NaOH. etc. are completely ionised in aqueous solution and have high values of conductivity (molar as well as equivalent). The weak electrolytes are ionised to a lesser extent in aqueous solution and have lower values of conductivity (molar as well as equivalent) .
TYPES OF SOLUTIONS
mymission.lamission.eduSubstances that dissolve in water to form ions are called electrolytes. The ions formed from these substances conduct electric current in solution, and can be tested using a conductivity apparatus (diagram below). Electrolytes are further classified as …
Conductance Measurements Part 1:Theory
www.currentseparations.comconductivity is symbolized by Λ, and it is defined as the solution con-ductivity (κ) normalized by the total ionic concentration (C): In this context, total ionic con-centration means “Molar concentra-tionofpositivecharges”(ornegative charges) in solution, expressed in units of mol/cm3. Strong vs.Weak Electrolytes In an ideal case, the ...
Conductivity Theory and Practice
www.tau.ac.ilWhat is a conductive solution? Conductivity is typically measured in aqueous solutions of electrolytes. Electrolytes are substances containing ions, i.e. solutions of ionic salts or of compounds that ionise in solution. The ions formed in solution are responsible for carrying the electric current. Electrolytes include acids,
ELECTRICAL CONDUCTIVITY
www.cerritos.eduMeasure the conductivity of each solution by using the conductivity probe. Record your observations. Now, in a clean small beaker fill it with 5 mL of 0.10 M H 2SO 4, place the conductivity probe into the solution and then slowly add the 0.10 M Ba(OH) 2 solution drop by drop. Mix well after the addition of each drop by gently swirling the beaker.
Specific Conductance: Theoretical Considerations and ...
pubs.usgs.gov1. Conductivity-temperature relationships for 0.01 N KG solution and 1 per mil chlorinity seawater 4 2. Percentage change in conductivity with temperature for 0.01 N KC1 solu tion and seawater in 1 °C increments 5 3. Values of the ratio of specific conductance to conductivity for 0.01 N KG solution and 1 per mil chlorinity seawater 6 4.
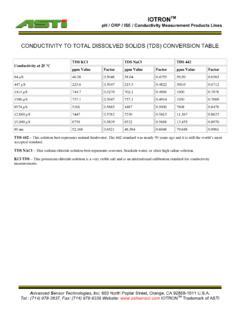
CONDUCTIVITY TO TOTAL DISSOLVED SOLIDS (TDS) …
www.astisensor.comTDS NACl – This sodium chloride solution best represents seawater, brackish water, or other high saline solution. KCl TDS – This potassium chloride solution is a very stable salt and is an international calibration standard for conductivity measurements. Total dissolved solids (TDS) units are computed from measured conductivity. The curves that