Enthalpies Of Formation
Found 6 free book(s)Standand Enthalpies of Formation & Standard Entropies of ...
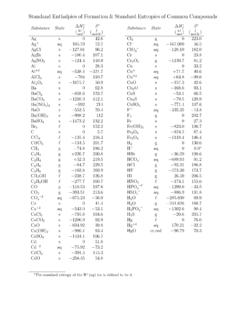
www.mrbigler.comStandand Enthalpies of Formation & Standard Entropies of Common Compounds Substance State ∆H f S (kJmol) (Jmol·K) Ag s 0 42.6 Ag+ aq 105.79 72.7 AgCl s −127.01 96.2
Standard Enthalpies of Formation
www.chemistry.alanearhart.orgStandard Enthalpies of Formation Alan D. Earhart 11/7/2016 Substance ΔH° f (kJ/mol) Substance ΔH° f (kJ/mol) Substance ΔH° f (kJ/mol) AgCl(s) -127.0 CaSO4(s) -1434.5 N 2H 4(g) +95.4 Al 2O 3(s)-1675.7 Fe 2O 3(s) -824.2 N 2H 4(l) +50.6 CHCl 3(g) -103.2 HBr(g) -36.4 N 2O(g) +82.1 CH 2Cl 2(g) -95.5 HCl(g) -92.3 N 2O 4(g) +9.1 CH 2O(g) -115.9 HF(g) -272.6 N
CALORIMETRY EXPERIMENT A ENTHALPY OF FORMATION …
jan.ucc.nau.edubetween the enthalpies of the products and the enthalpies of the reactants. In other words, it is the change in energy for a given amount of a given reaction. The enthalpy of formation, H f is defined as the enthalpy or heat change that results when one mole of a compound is formed from its elements. The standard enthalpy of formation is defined
Yu-ran Luo
staff.ustc.edu.cnof formation ∆ f Ho of a large number of atoms, free radicals, ions, clusters and compounds is available from the websites of NIST, NASA, CODATA, and IUPAC . Most authors prefer to use the BDE values at 298 .15 K . The following seven tables provide essential information of ex-perimental BDE values of R−X and R+−X bonds .
Standard Enthalpy of Formation* for Various Compounds
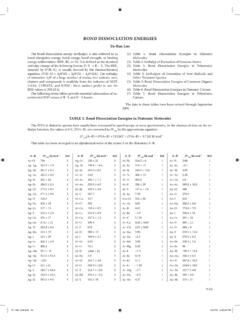
www.nshs-science.netStandard Enthalpy of Formation* for Atomic and Molecular Ions Cations ΔH˚ f (kJ/mol) Cations ΔH˚ f (kJ/mol) Anions ΔH˚ f (kJ/mol) Anions ΔH˚ f (kJ/mol) Ag+(aq) +105.9 K+(aq) −251.2 Br−(aq) −120.9 H 2PO 4 −(aq) −1302.5 Al3+(aq) −524.7 Li+(aq) −278.5 Cl−(aq) −167.4 HPO 4 2−(aq) −1298.7 Ba2+(aq) −538.4 Mg2+(aq) −462.0 ClO
Enthalpy of Formation of Magnesium Oxide
www.webpages.uidaho.eduEnthalpy of Formation of Magnesium Oxide Adapted with permission from the United States Air Force Academy. Revised: 2013. BACKGROUND In this laboratory, we will introduce one of the most often used techniques in thermochemistry, calorimetry. Although we often think of calorimetry in terms of finding the number of calories in a