Montelukast 4 mg chewable tablets
Found 7 free book(s)Singulair (Montelukast Sodium)
www.accessdata.fda.govSINGULAIR ® XXXXXXX (Montelukast Sodium) Tablets, Chewable Tablets, and Oral Granules to the 4-mg chewable tablet, it can also be used as an alternative formulation to the 4-mg chewable tablet
MONTELUKAST 4 MG CHEWABLE TABLETS …
www.mhra.gov.ukThe MHRA granted Caduceus Pharma Ltd Marketing Authorisations (licences) for the medicinal products Montelukast 4 mg and 5 mg chewable tablets on …
Proposed Prescribing Information (USPI) Singulair
www.merck.com3 For pediatric patients 2 to 5years of age: one 4-mg chewable tablet or one packet of 4-mg oral granules. For pediatric patients 6to 23months of age: one packet of 4-mg …
All of the following medications are gluten free …
www.glutenfreedrugs.comAll of the following medications are gluten free unless otherwise noted Generic drugs can be produced from many manufacturers and not all manufacturers use the
NIHS 医薬品安全性情報 Vol.13 No.02 201501//29
www.nihs.go.jp国立医薬品食品衛生研究所 安全情報部. 目 次 http://www.nihs.go.jp/dig/sireport/index.html 各国規制機関情報 【米. FDA(U. S. Food and Drug Administration)】
NDA 20-829, 20-830, 21-409 Merck Singulair™ …
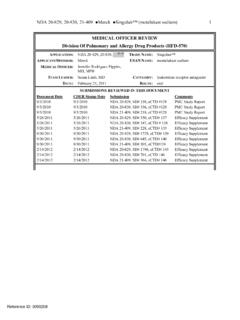
www.fda.gov(b) (4) NDA 20-829, 20-830, 21-409 Merck Singulair™ (motelukast sodium) 1 MEDICAL OFFICER REVIEW Division Of Pulmonary and Allergy Drug Products (HFD-570)
Drugs approved from 1st Jan 2014 to 31 december …
cdsco.nic.inDrugs approved from 1st Jan 2014 to 31 december 2014 S.No Drug Indication Date For the treatment of overactive bladder with Tolterodine Tartrate Extended release symptoms of …