Standard enthalpies of formation
Found 7 free book(s)Thermodynamics
www.chem.tamu.eduStandard reaction enthalpy (∆Ho) refers to reactions where all products and reactants are in their standard state . Definitions of Standard States • For a gas the standard state is a pressure of exactly 1 atmosphere. ... Standard Enthalpies of Formation .
Spontaneous Processes and Spontaneity, Entropy, Free Energy
ww2.odu.eduStandard Entropies and the Third Law of Thermodynamics • The standard entropyof a substance or ion (Table 19.1), also called its absolute entropy, So, is the entropy value for the standard state of the species. • Note that the elements have nonzero values, unlike standard enthalpies of formation, ∆Hfo, which by convention, are zero.
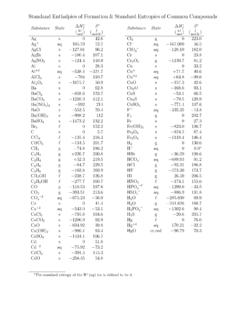
Standand Enthalpies of Formation & Standard Entropies of ...
www.mrbigler.comStandand Enthalpies of Formation & Standard Entropies of Common Compounds Substance State ∆H f S (kJmol) (Jmol·K) Ag s 0 42.6 Ag+ aq 105.79 72.7 AgCl s −127.01 96.2
CALORIMETRY EXPERIMENT A ENTHALPY OF FORMATION …
jan.ucc.nau.edubetween the enthalpies of the products and the enthalpies of the reactants. In other words, it is the change in energy for a given amount of a given reaction. The enthalpy of formation, H f is defined as the enthalpy or heat change that results when one mole of a compound is formed from its elements. The standard enthalpy of formation is defined
© www.CHEMSHEETS.co.uk 10-Mar-2016 Chemsheets A2 …
mchem.weebly.com1) Calculations involving enthalpies of formation (“Type 1 questions”) If the enthalpy of formation for the reactants and products in a reaction are known, the overall enthalpy change is easy to calculate. H = [SUM of f H products] – [SUM f H reactants] Remember that f H of all elements in their standard …
Standard Enthalpies of Formation - chemistry.alanearhart.org
www.chemistry.alanearhart.orgStandard Enthalpies of Formation Alan D. Earhart 11/7/2016 Substance ΔH° f (kJ/mol) Substance ΔH° f (kJ/mol) Substance ΔH° f (kJ/mol) AgCl(s) -127.0 CaSO4(s) -1434.5 N 2H 4(g) +95.4 Al 2O 3(s)-1675.7 Fe 2O 3(s) -824.2 N 2H 4(l) +50.6 CHCl 3(g) -103.2 HBr(g) -36.4 N 2O(g) +82.1 CH 2Cl 2(g) -95.5 HCl(g) -92.3 N 2O 4(g) +9.1 CH 2O(g) -115.9 HF(g) -272.6 N
Standard Enthalpy of Formation* for Various Compounds
www.nshs-science.netStandard Enthalpy of Formation* for Atomic and Molecular Ions Cations ΔH˚ f (kJ/mol) Cations ΔH˚ f (kJ/mol) Anions ΔH˚ f (kJ/mol) Anions ΔH˚ f (kJ/mol) Ag+(aq) +105.9 K+(aq) −251.2 Br−(aq) −120.9 H 2PO 4 −(aq) −1302.5 Al3+(aq) −524.7 Li+(aq) −278.5 Cl−(aq) −167.4 HPO 4 2−(aq) −1298.7 Ba2+(aq) −538.4 Mg2+(aq) −462.0 ClO