Search results with tag "Influenza vaccine"
07.265 Quadrivalent Inactivated Influenza Vaccine (QIV ...
albertahealthservices.cainactivated influenza vaccine or quadrivalent live attenuated influenza vaccine can be used as long as there is a minimum interval of 4 weeks between doses. If a child receives a dose of trivalent inactivated influenza vaccine as their first dose, q uadrivalent inactivated influenza vaccine can be administered as the second dose.
Vaccination of the egg-allergic individual
www.allergy.org.auegg ovalbumin in the pandemic and seasonal inactivated influenza vaccines to less than 1 ug of egg protein per vaccine dose. • Seasonal inactivated influenza vaccine (s) • Pandemic inactivated influenza vaccine(s) (e.g. H1N1, bird or swine flu vaccines). • Yellow Fever vaccine (important for travelers and those living in an endemic area)
Recommended composition of influenza virus vaccines for ...
www.who.intFeb 26, 2021 · for inclusion in influenza vaccines. 2. for the northern and southern hemisphere influenza seasons, respectively. This recommendation relates to the influenza vaccines for use in the northern hemisphere 2021-2022 influenza season. A recommendation will be made in September 2021 relating to vaccines
How to Administer Intramuscular and Intranasal Influenza ...
www.immunize.orgInactivated Influenza Vaccines (IIV), including recombinant hemagglutinin (RIV), cell culture-based vaccine (ccIIV), adjuvanted influenza vaccine (aIIV), and egg culture-based inactivated influenza vaccines. 1 Use a needle long enough to reach deep into the muscle.
2020-2021 Vaccine Label Examples - Centers for Disease ...
www.cdc.govSep 14, 2021 · 1 2021–2022 Influenza Season Vaccine Label Examples Healthcare professionals can easily confuse different influenza vaccines within the storage unit. Labeling the area or container where vaccines are stored can help staff quickly locate and choose the correct vaccine—perhaps preventing a vaccine
2018/19 Seasonal Influenza Vaccine Eligibility
www.bccdc.ca2018/19 Seasonal Influenza Vaccine Eligibility . Trivalent and Quadrivalent Inactivated Influenza Vaccines (TIIV & QIIV), and Quadrivalent Live Attenuated Influenza Vaccine (LAIV-Q)
Recommended Adult Immunization Schedule 2021 for ages …
www.cdc.govInfluenza vaccine (inactivated) IIV: Many brands. Influenza vaccine (live, attenuated) LAIV4. FluMist® Quadrivalent: Influenza vaccine (recombinant) RIV4: Flublok® Quadrivalent. Measles, mumps, and rubella vaccine: MMR. M-M-R II® Meningococcal serogroups A, C, W, Y vaccine. MenACWY-D: MenACWY-CRM. MenACWY-TT. Menactra® Menveo® MenQuadfi ...
2021-2022 Influenza Vaccination Recommendations and ...
emergency.cdc.gov•Influenza vaccines expected to be available for the 2021-22 season. •U.S. influenza vaccine viral composition for the 2021-22 season. •Change in FDA-approved age indication for Flucelvax Quadrivalent from ≥4 years to ≥2 years. •Timing of Vaccination language. •Co-administration of influenza and COVID-19 vaccines.
CONSENT FORM FOR SEASONAL INFLUENZA VACCINE
ahfbaltic.comInsert Facility Logo CONSENT FORM FOR SEASONAL INFLUENZA VACCINE I have read or have had explained to me the information about influenza and influenza vaccine.
VACCIE IFORMATIO STATEMET Influenza (Flu) …
www.immunize.orgTitle: Vaccine Information Statement: Inactivated Influenza Vaccine Author: CDC/NCIRD Subject: Vaccine Information Statement: Inactivated Influenza Vaccine
Guideline on Influenza Vaccines - European Medicines Agency
www.ema.europa.euinfluenza vaccines as detailed in the scope of this module. Specific requirements for adjuvanted vaccines or live attenuated vaccines are exemplified in dedicated paragraphs as appropriate. For further details, this section should be c omplemented with the principles outlined in the guidelines listed
Guillain-Barré Syndrome and Influenza Vaccine
www.who.intConclusions (1) • A/NJ/76 (H1N1) influenza vaccine associated with increased risk of GBS in adults 6 - 8 weeks following vaccine in US civilians • Reasons unknown • No clear biological explanation • Data on risk of GBS following other swine-antigen containing vaccines too limited to allow for conclusions
Many vaccine information statements are Influenza (Flu ...
www.cdc.govInfluenza vaccine can prevent influenza (flu). Flu is a contagious disease that spreads around the United States every year, usually between October and May. Anyone can get the flu, but it is more dangerous for some people. Infants and young children, people
WyIR 2020-2021 Influenza Vaccine Cheat Sheet
health.wyo.govInfluenza vaccine, quadrivalent, adjuvanted ≥65 yrs; 205 70461-0120-03; 70461-0120-04 90694; SEQ; Author: Kristy Westfall Created Date: 10/19/2020 9:22:00 AM ...
Contraindications
www.who.intContraindications for use (of CAIV-T influenza vaccine) include anaphylactic reactions to eggs, a history of Guillain-Barré syndrome, patients aged <18 years on long-term aspirin therapy, pregnancy during the first trimester, and various states of immunosuppression. Page 285 2005 Influenza vaccines (WHO position paper) Weekly Epid.
standing orders for Administering Influenza Vaccine …
www.immunize.organd recommended influenza vaccine (i.e., any IIV or RIV) that is otherwise appropriate for the patient’s age and . health status. For people with a history of severe allergic reaction to egg involving any symptom other than hives
Updated summary of risk management plan for Comirnaty ...
medsafe.govt.nzThe Comirnaty data sheet, consumer medicine information and the package leaflet give essential information for healthcare professionals and patients on how to use the vaccine. ... influenza vaccine) Safety and immunogenicity of BNT162b2 and quadrivalent seasonal influenza
Standing Orders for Administering Influenza Vaccine to Adults
www.immunize.orgFemale or male 130–152 lbs 22–25 1" Deltoid muscle of arm Female 153–200 lbs 22–25 1–1½" Deltoid muscle of arm Male 153–260 lbs 22–25 1–1½" Deltoid muscle of arm Female 200+ lbs 22–25 1½" Deltoid muscle of arm Male 260+ lbs 22–25 1½" Deltoid muscle of arm 4 Prepare to Administer Vaccine
Commonly Used Spanish Patient Forms: Consent, Refusal, …
www.cigna.comConsent to Immunization - Adult . GI Consent to Operation or Other Medical Services . Consent to Photograph . Consent for Depo-Provera . Important Information about Influenza and Influenza Vaccine . Consent to Medical Treatment of a Minor . Outpatient Surgery Consent to Operation or Other Medical Services . Informed Consent for Psychotropic ...
Guide for Determining the Number of Doses of …
www.immunize.orgGuide for Determining the Number of Doses . of Influenza Vaccine to Give to Children Age 6 Months Through 8 Years During the 2017–2018 Influenza Season
PCV13 vs PPSV23: Which to Give and When
www.medicine.wisc.eduACIP January 2013 Updated Immunization Guidelines • Live attenuated influenza vaccine: will include four strains for the 2013‐2014 flu season; one A
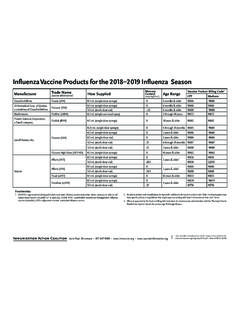
Influenza Vaccine Products for the 2021-2022 Influenza …
www.immunize.orgTitle: Influenza Vaccine Products for the 2021-2022 Influenza Season Author: IAC Keywords: influenza vaccine products for the 2021 2022 influenza season, easy to read chart the displays influenza vaccine products for the 2021 2022 influenza season, coding information for influenza vaccines for the 2021 2022 season, what vaccines are available for the 2021 2022 …
Vaccine Information Statement: Inactivated Influenza Vaccine
www.immunize.orginfluenza vaccine (the flu shot). Young children who get the flu shot along with pneumococcal vaccine (PCV13) and/or DTaP vaccine at the same time might be slightly more likely to have a seizure caused by fever. Tell your health care provider if a child who is getting flu vaccine has ever had a seizure.
Influenza: Questions and Answers
www.immunize.orgSome influenza vaccines are made by growing influ-enza viruses in eggs, then inactivating (killing and dis-rupting) the viruses and purifying the vaccine to remove almost all of the egg protein. These egg-cul-ture-based vaccines are given as an intramuscular injection. Two other influenza vaccines (cell-culture
Vaccine Information Statement: Inactivated Influenza Vaccine
www.immunize.orgVaccine Information Statement • Influenza, Inactivated (8/6/21)• Arabic Translation • Distributed by the Immunization Action Coalition Influenza …
Vaccine Information Statement: Inactivated Influenza Vaccine
www.cdc.govInfluenza vaccine can prevent influenza (flu). Flu is a contagious disease that spreads around the United States every year, usually between October and May. Anyone can get the flu, but it is more dangerous for some people. Infants and young children, people 65 years and older, pregnant people,
Influenza A+B FIA - Quidel
www.quidel.comPage 3 of 3 Will the Sofia Influenza A+B FIA show a positive test result after someone has had a nasally administered vaccine? Individuals who received nasally administered influenza vaccine may have a positive flu A and/or flu B test result
Influenza Vaccine Payment Allowances - Annual Update for ...
www.cms.govSep 08, 2021 · Influenza Vaccine Payment Allowances - Annual Update for 2021-2022 Season . MLN Matters Number: MM12421 . Related CR Release Date: September 8, 2021 . Related CR Transmittal Number: R10983CP . Related Change Request (CR) Number: 12421 . Effective Date: August 1, 2021 . Implementation Date: No later than October 1, 2021 . Provider Types Affected
Influenza Vaccine Products for the 2017-2018 …
immunize.org3. Live attenuated influenza vaccine (LAIV4; FluMist) is not recommended by CDC’s Advisory Committee on Immunization
Similar queries
Quadrivalent Inactivated Influenza Vaccine, Influenza vaccine, Q uadrivalent inactivated influenza vaccine, Seasonal, Influenza vaccines, Influenza, Vaccines, Recommended composition of influenza virus vaccines, Vaccine, Vaccine Label Examples, CONSENT FORM FOR SEASONAL INFLUENZA VACCINE, Information, Vaccine Information Statement, On Influenza Vaccines, H1N1, Risk management plan, Consumer, Spanish, Consent, For Determining the Number of Doses, Children, PCV13 vs PPSV23: Which to Give and, Influenza vaccine products, DTaP vaccine, Influenza: Questions and Answers, Influ-enza, Immunization Action Coalition Influenza, Vaccine Information, Influenza A+B FIA, Influenza Vaccine Products for the 2017-2018