Carboxylic Acid Structure and Chemistry
amines, alcohols, phenols, aldehydes, ketones, esters, amides and isosteric compounds. Carboxylic acids have a greater number of dipoles and stronger dipoles than these other organic compounds, and thus can form more and stronger H-bonds with other substances capable of H-bonding interactions. The dipolar nature of these other organic functional
Tags:
Acid, Carboxylic, Carboxylic acids, Aldehydes
Information
Domain:
Source:
Link to this page:
Please notify us if you found a problem with this document:
Documents from same domain
HALOGENATED HYDROCARBON STRUCTURE AND CHEMISTRY
webhome.auburn.eduPrinciples Of Drug Action 1, Spring 2005, Halogenated Hydrocarbons 3 Note in these examples that the halogen atom is merely one of the substituent groups of the
THYROID HORMONE TUTORIAL: THE THYROID AND …
webhome.auburn.eduEndocrine Pharmacotherapy Module: Thyroid Section, Summer, 2001 6 D. Coupling of lodotyrosine Residues. This reaction takes place at thyroglobulin and involves the coupling of two DIT residues or one
THYROID HORMONE TUTORIAL: THYROID PATHOLOGY Jack …
webhome.auburn.eduEndocrine Module (PYPP 5260), Thyroid Section, Spring 2002 1 THYROID HORMONE TUTORIAL: THYROID PATHOLOGY Jack DeRuiter I. INTRODUCTION Thyroid disorder is a general term representing several different diseases involving thyroid
HisTrap HP, 1 ml and 5 ml - webhome.auburn.edu
webhome.auburn.eduInstructions 71-5027-68 AF HisTrap affinity columns GE Healthcare HisTrap HP, 1 ml and 5 ml HisTrap™ HP is a ready to use column, prepacked with precharged Ni Sepharose™ High Performance. This prepacked column is ideal for preparative purification
Electron Paramagnetic Resonance Theory E. Duin
webhome.auburn.eduspectrum of a single crystal you would have to measure a spectrum for the crystal in all possible x-, y-and z-directions. Alternatively you could ground up the single crystal into an actual powder. Fig. 6: Dependency of the g value on the oritentation of the molecules in the magnetic field.
Field, Theory, Crystal, Electron, Resonance, Paramagnetic, Electron paramagnetic resonance theory
Save the Ferrets Curriculum - Auburn University
webhome.auburn.eduOct 24, 2016 · Save the Ferrets Introduction WebQuest (30 minutes) 3. Introduction to Storyboard poster (15 minutes) 4. Storyboard about Ferrets (15 minutes) Background for the Teacher The Black Footed Ferret, Mustela nigripes In the prairies and grasslands of the midwestern United States, there is a small, nocturnal, creature who lives in the burrows of ...
AMIDES AND RELATED FUNCTIONAL GROUPS
webhome.auburn.eduderivatives of carboxylic acids in which the -OH of the acid has been replaced by -NR2 where R= H, alkyl, aryl, etc.): Like amines, amides can be classified as "primary", "secondary" or "tertiary" depending on the degree of carbon substitution on nitrogen: Amides may also be sub-classified as aliphatic, aromatic (i.e. anilides or benzamides) or ...
Media, Acid, Derivatives, Carboxylic, Derivatives of carboxylic
General Protocol for Precipitation of DNA with Sodium ...
webhome.auburn.eduGeneral Protocol for Precipitation of DNA with Sodium Acetate and Ethanol For ethanol precipitation of DNA from solution, the solution needs to have a high
General, Protocol, Precipitation, General protocol for precipitation of dna
Related documents
Identification of an Unknown – Alcohols, Aldehydes, and ...
people.chem.umass.edu2,4-Dinitrophenylhydrazine : Aldehydes and ketones react with 2,4-dinitrophenylhydrazine reagent to form yellow, orange, or reddish-orange precipitates, whereas alcohols do not react. Formation of a precipitate therefore indicates the presence of an aldehyde or ketone. The precipitate from this test also serves as a solid derivative.
Safety Data Sheet
newpig.scene7.comketones, aldehydes and other unidentified organic compounds. Unusual Hazards: Refer to absorbed liquid(s) SDS(s). The Universal PIG Absorbents do not render liquids nonflammable, neutral or less hazardous. 6. Accidental Release Measures If material is unused, sweep or pick up and dispose of as a non hazardous material. 7. Handling and Storage None
Sheet, Data, Safety, Safety data sheet, Aldehydes, Aldehydes and
Carbonyl Chemistry (12 Lectures)
www.ch.ic.ac.uk¥Aldehydes and ketones are hydrogen bond acceptors; this makes them have considerable solubilities in water. R C R ' O O H H H O H Ketones such as acetone are good solvents because they dissolve both aqueous and organic compounds Recall that …
Lecture, Chemistry, Aldehydes, Carbonyl, Carbonyl chemistry, Lecture 12, Aldehydes and
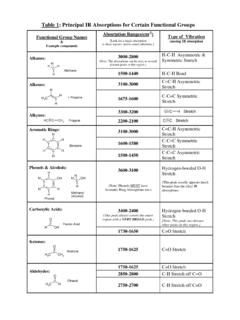
Table 1: Principal IR Absorptions for Certain Functional ...
academics.wellesley.eduAldehydes: H C 3 C H O Ethanal 2750-2700 C-H Stretch off C=O . Table 1: Principal IR Absorptions for Certain Functional Groups Functional Group Names & Example compounds Absorption Ranges(cm-1) [Look for a single absorption in these regions, unless stated otherwise.] Type of Vibration
Oxidation Reactions of Sugars
butane.chem.uiuc.eduAldehydes are much easier to oxidize than ketones. The oxidizing agents Ag+, Cu+2, and K 2CrO 4 can transform an aldehyde to a carboxylic acid. The carbonyl carbon is oxidized by 2 electrons from the I to the III oxidation state. Silver metal is …
CERTIFICATE - images.thdstatic.com
images.thdstatic.comTotal Aldehydes (B)-0.043 ppm 4-Phenylcyclohexene 4994-16-5 6.5 µg/m³ Particle Matter less than 10 µm (C)-20 µg/m³ 1-Methyl-2-pyrrolidinone (D) 872-50-4 160 µg/m³ Individual VOCs (E)-1/2 CREL or 1/100th TLV-(A) Defined to be the total response of measured VOCs falling within the C6 – C16 range, with responses calibrated to a toluene ...
Structure Determination of Organic Compounds
bionmr.unl.eduErno Pretsch¨ · Philippe Buhlmann¨ · Martin Badertscher Structure Determination of Organic Compounds Tables of Spectral Data Fourth, Revised and Enlarged Edition 123
COVID-19 CHEMICAL DISINFECTANT SAFETY INFORMATION
www.ehs.washington.edu201 Hall Health Center, Box 354400, Seattle, WA 98195- 4400 206.543.7262 ᅵ fax 206.543.3351ᅵwww.ehs.washington.edu COVID-19 CHEMICAL …
Information, Chemical, Safety, Disinfectants, 19 chemical disinfectant safety information, 19 chemical
CHEMISTRY Module 1 Fundamentals of Chemistry
sites.ntc.doe.govREFERENCES DOE-HDBK-1015/1-93 Fundamentals of Chemistry REFERENCES Donald H. Andrews and Richard J. Kokes, Fundamental Chemistry, John Wiley & Sons,