Transcription of Chapter 11: Nucleophilic Substitution and Elimination ...
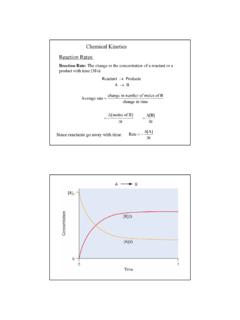
1 1 Chapter 11: Nucleophilic Substitution and EliminationWalden InversionOOHOHHOO(S)-(-) Malic acid[a]D= PCl5 OClOHHOOAg2O, H2 OOOHOHHOO(R)-(+) Malic acid[a]D= + PCl5Ag2O, H2 OOClOHHOO(+)-2-Chlorosuccinic acid(-)-2-Chlorosuccinic acidThe displacement of a leaving group in a Nucleophilic substitutionreaction has a defined stereochemistryStereochemistry of Nucleophilic substitutionp-toluenesulfonate ester (tosylate): converts an alcohol intoa leaving group; tosylate are excellent leaving as TosCXNu:CNu+ X-X= Cl, Br, ICOHSOOClCH3+COSOOCH3tosylateCOSOOCH3Nu: CNuSOO-OCH3+2 The Nucleophilic Substitution reaction inverts the Stereochemistry of the carbon (electrophile)- Walden inversionOHTos-ClpyridineOHHTos[a]D= + [a]D= + [a]D= + TosO -HO-HOH[a]D= [a]D= - +[a]D= of Nucleophilic substitutionReaction rate: how fast (or slow) reactants are converted into product( kinetics )Reaction rates are dependent upon the concentration of the reactants.
2 ( reactions rely on molecular collisions)Consider:At a given temperature:If [OH-] is doubled, then the reaction rate may be doubledIf [CH3-Br] is doubled, then the reaction rate may be doubledA linear dependence of rate on the concentration of two reactants iscalled a second-order reaction (molecularity)CBrHHHHO _CHOHHHBr _3 CBrHHHHO _CHOHHHBr _Reaction rates (kinetic) can be expressed mathematically:reaction rate = disappearance of reactants (or appearance of products)For the disappearance of reactants:rate = k [CH3Br] [OH-][CH3Br] = CH3Br concentration[OH-] = OH- concentrationk= constant (rate constant)For the reaction above, product formation involves a collision between both reactants, thus the rate of the reaction is dependent upon the concentration of secNucleophilic Substitution comes in two reaction types:SN2 SN1 S= substitutionS= Substitution N= nucleophilicN= Nucleophilic 2= biomolecular1= unimolecular rate = k [R-X] [Nu:]rate = k [R-X]4 The SN2 Reaction: MechanismSteric effects in the SN2 reaction.
3 For an SN2 reaction, the nucleophile approaches the electrophilic carbon at an angle of 180 from the leaving group (backside attack) the rate of the SN2 reaction decrease as the steric hindrance ( Substitution ) of the electrophile <<<<realtivereactivity<< 1150040,0002,000,000 Increasing reactivity in the SN2 reactionVinyl and aryl halides do not react in nucleophilc Substitution reactionsXR1R2R3+ Nu:R1 NuR2R3XX+ Nu:XNuXR1R2R3+ (CH3)2 CuLiCH3R1R2R3 From Chapter 10: NOT a nucleophilcsubstitution reactionThe nature of the nucleophile in the SN2 Reaction:The measure of nucleophilicity is : + R-X Nu-R + X:__Nu: + R-X Nu-R + X:_+Nu + H3C-Br Nu-CH3 + Br -Nu = H2O relative reactivity= 1CH3CO2- 500NH3 700Cl- 1,000HO- 16,000CH3O- 25,000I-100,000N C-125,000HS-125,0006 Nucleophiles are Lewis bases Nucleophilicity roughly parallels basicity when comparing nucleophilesthat have the same attacking atomNu: CH3O- HO- CH3CO2- H2 Orelative reactivity.
4 25,000 16,000 500 1pKa of the conj. acid: usually increases going down a column of the periodicchart. Thus, sulfur nucleophiles are more reactive than oxygennucleophiles. I- > Br- > Cl-. Negatively charges nucleophiles are usually more reactive than neutralnucleophiles. The role of the leaving group in SN2 reactions :CXHHHNu _CNuHHHX _CXHHHNu _CHOHHH+X++The leaving group is usually displaced with a negative chargeThe best leaving groups are those with atoms or groups that can best stabilize a negative leaving groups are the conjugate bases of strong acidsH-X H+ + X-the lower the pKa of H-X, the stronger the , H2N-, RO- F- Cl- Br- I- TosO- <<1 1 200 10,000 30,000 60,000 >15 :Relative Reactivity.
5 Increasing reactivity in the SN2 reactionpKa:CXHHHNu _CNuHHHX _+CXHHHNud-d- Charged Leaving Groups: conversion of a poor leaving group to a good onepKa ofH3O+= _CNuHHH+HH+OH2 COHHHHH+The role of the solvent in SN2 reactions :Descriptor 1:polar: high dipole momentnon-polar: low dipole moment (hexanes)Descriptor 2:protic: acidic hydrogens that can form hydrogen bonds(water, alcohols)aprotic: no acidic hydrogensHHOSO+_d +d _d +RHOd _d +wateralcoholsDMSOSO+_DMSO[(CH3)2N]3PO_+ hexamethylphosphoramide(HMPA)CH3 CNacetonitrileHNOCH3CH3dimethylformamide (DMF)8 Solvation: solvent molecules form shells around reactants anddramatically influence their reactivity.
6 X_HHOd _HHOHHOHHOHHOHHOHHOd _d _d _d +d +d +d +d +Y+HHOHHOHHOHHOHHOHHOd +d +d +d +d +d +d _d _d _d _Polar protic solvents stabilize the nucleophile, thereby lowering its energy. This will raise the activation energy of the reactions are not favored by polar protic aprotic solvents selectively solvate cations. This raises the energy of the anion (nucleophile), thus making it more reactive. SN2 reactions are favored by polar aprotic + N3- CH3CH2CH2CH2CH2Br + Br- Solvent: CH3OH H2O DMSO DMF CH3CN HMPA relativereativity: 1 7 1,300 2,800 5,000 200,000DE DE 9 The SN1 Reaction: kinetics : first order reaction (unimolecular)rate = k [R-X] [R-X]= alkyl halide nucleophile does not appear in the rate expression-changing the nucleophile concentration does not affectthe rate of the reaction!
7 Must be a two-step reactionThe overall rate of a reaction is dependent upon the sloweststep: rate-limiting stepThe Mechanism of the SN1 Reaction10step 1DG step 2DG step 1 >> DG step 2DG step 1 is rate-limitingReactivity of the Alkyl Halide in the SN1 Reaction:Formation of the carbocation intermediate is , carbocation stability greatly influence the (1 )CRHRS econdary (2 )CRRRT ertiary (3 )<<+++<CCC+Allylic+CHHH+MethylBenzylic_~ _~Increasing carbocation stabilityThe order of reactivity of the alkyl halide in the SN1 reactionexactly parallels the carbocation stability11 Allylic and benzylic carbocations are stablized by resonance Stereochemistry of the SN1 Reaction: it is actually a complicated issue.
8 For the purpose of Chem 220a, sect. 2 the stereochemistry of the SN1 reaction gives racemization. A chiral alkyl halide willundergo SN1 Substitution to give a racemic productCH2CH2CH2CH3 ClH3CH2CH3CH2 OCH2CH2CH2CH3H3CH3CH2 CCarbocation is achiralOH2OH2CH2CH2CH2CH3 OHH3CH2CH3 CCH2CH2CH2CH3 OHH3CH2CH3 Cenantiomers: produced in equal amount12 The role of the leaving group in SN1 reactions : same as for the SN2 reactionTosO- > I- > Br- > Cl- ~ H2O_Charged Leaving Groups: conversion of a poor leaving group to a good oneCORRRHH+CORRRHH+-H2 ORCRR+CNuRRRNu:The role of the nucleophile in SN1 reactions .
9 NoneInvolvement of the nucleophile in the SN1 reaction is after the rate-limiting step. Thus, the nucleophile does not appear in the rate expression. The nature of the nucleophile plays no role in the rate of the SN1 role of the solvent in SN1 reactions :polar solvents are fovored over non-polar for the SN1 reactionprotic solvents are favored over aprotic for the SN1 reactionSolvent polarity is measured by dielectric constant (e)Hexane e = (CH3CH2)2O 80proticaproticnonpolarpolar13 CClCH3CH3H3C+ ROHCORCH3CH3H3C+ HClSolvent: Ethanol 40% H2O 80% H2O H2O60% Ethanol 20% EthanolRel.
10 Reactivity: 1 100 14,000 100,000 Increasing reactivity in the SN1 reactionCRRRClCRRRCld _d +HHOHHOHHOHHOHHOHHO sp3tetrahedralSolvent stablization of the intermediatesSolvent stablization of the transition stateCl_HHOHHOHHOHHOd +d +d +d +C+HHOHHOHHOHHOHHOHHOd _d _d _d _d _d _sp2trigonal planarStabilization of the intermediate carbocation and the transition state by polar protic solvents in the SN1 reaction14 Elimination reactions : Nucleophiles are Lewis bases. In the reactionwith alkyl halides, they can also promote Elimination reactionsrather than Substitution .