Transcription of CHE 31. INTRODUCTION TO CHEMICAL ENGINEERING …
1 CHE 31. INTRODUCTION TO CHEMICAL ENGINEERING CALCULATIONSL ecture 11 combustion ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os LECTURE 11. combustion ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE2A combustion ProcessLECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE3 CHEMICAL ReactiionsAssociated with combustion ProcessesC + O2========>CO2C + >CO2H + >H2OS + O2========>SO2 LECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE4 Terms Associated with combustion ProcessesOrsatAnalysisRefers to the type of gas analysis which eliminates water as a component (dry-free basis).
2 If water is included in the report, it is termed wet-basis AirThe amount of air required for complete combustion of C, H, and S. It does not depend on how much material is actually but what can be AirThe amount of air in excess of that required for complete combustion . The % excess air is the same as % excess 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE5 Example 11-1. Theoretical and Stoichiometric AirIn a given process, 100 kmol of carbon is burned in a furnace. It has been found that 20% of the carbon undergoes incomplete combustion resulting to CO production. The rest of the carbon undergoes complete combustion . Determine the amount of air required (in kmol) if 50% excess O2must be reactions :C + O2========>CO2C + >COLECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING .
3 University of the Philippines Los Ba os SLIDE6 Example 11-1. Theoretical and Stoichiometric AirCalculate for theoretical O2needed:Assume that all the carbon is burned completely to kmol C (1/1) = 100 kmol O2It is not correct to do the following:C CO2:100 kmol C ( )(1/1) = 80 kmol O2C CO:100 kmol C ( )( ) = 10 kmol O2 LECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE7 Example 11-1. Theoretical and Stoichiometric AirTotal O2required stoichiometricallybased on the actual process:Stoichiometric O2= (80 + 10) kmol = 90 kmolTheoretical O2is based not on what is stoichiometricallyneeded according to what is actually Air = (100 kmol)(1 ) = kmolAnd the actual air supplied:Actual Air = kmol ( ) = kmolLECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING .
4 University of the Philippines Los Ba os SLIDE8 Example 11-2. combustion of Propane (C3H8)Fuels for motor vehicles other than gasoline are being eyed because they generate lower levels of pollutants than does propane (C3H8) has been suggested as a source of economic power for vehicles. Suppose that in a test, 20 kg of C3H8is burned with 400 kg of air to produce 44 kg of CO2and 12 kg of the percent excess 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE9 Example 11-2. combustion of Propane (C3H8)Write the overall combustion reaction for the fuel assuming it is burned completely:C3H8+ 5O2========>3CO2+ 4H2 OFor 20 kg of C3H8, the theoretical O2required is:38238238381kmol C H5 O20 kg C H= kmol kg C H1C H LECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING .
5 University of the Philippines Los Ba os SLIDE10 Example 11-2. combustion of Propane (C3H8)The actual O2supplied is221kmol air1air400 kg air= kmol O29 kg The percent excess air (or O2) kmol O kmol O%excess air = 100=28% kmol OLECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE11 Example 11-3. combustion of Methane (CH4)Generation of methane-rich biogas is a way to avoid high waste-disposal costs, and burning it can meet up to 60% of the operating costs for such waste-to-energy the complete combustion of kg of methane (CH4) in biogas with 300 kg of air. Determine the % excess of air, and the total moles and composition of the flue 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE12 Example 11-3.
6 combustion of Methane (CH4)Degrees of Freedom Analysis: Atomic BalanceUnit: Reactorunknowns (P,x1,x2,x3,x4)+5independentatomic specie(s)independent nonreactivemolecularspecie(s)other equations:Degreesof freedom0 LECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE13 Example 11-3. combustion of Methane (CH4)Write the atomic species balances (mole basis):(1)C:16 kg CH4(1/16)(1) = Px1(2) H:16 kg CH4(1/16)(4) = Px4(3) O:300 kg Air (1/29)( )(2) = 2Px2+ 2Px1+ Px4(4) N:300 kg Air (1/29)( )(2) = 2Px3(5) x:x1+ x2+ x3+ x4= 1 LECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE14 Example 11-3. combustion of Methane (CH4)Simplifying the equations(1)C:1 = Px1(2) H:4 = Px4(3) = 2Px2+ 2Px1+ Px4(4) = 2Px3(5) x:x1+ x2+ x3+ x4= 1 LECTURE 10.
7 Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE15 Example 11-3. combustion of Methane (CH4)If composition of flue gas is expressed in terms of actual number of moles (n s) instead of mole fractions (x s)C:1 = n1H:4 = n4 = 2n2+ 2n1+ n4 = 2n3 n:n1+ n2+ n3+ n4= PLECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE16 Example 11-3. combustion of Methane (CH4)Solving for the n sand P:n1= 1 kmol CO2n2= kmol O2n3= 2 kmol H2On4= kmol N2P = kmolSolving for the mole fractions:x1= (1 ) = kmol CO2/kmol Px2= ( ) = kmol O2/kmol Px3= ( ) = kmol N2/kmol Px4= (2 ) = kmol H2O/kmol PLECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE17 Example 11-3.
8 combustion of Methane (CH4)Solving for % excess air:Write the overall combustion reaction for the fuel assuming it is burned completely:CH4+ 2O2========>CO2+ 2H2 OFor 16 kg of C3H8, the theoretical air required is:4244821kmol CH2 O1 Air29 kg Air16 kg CH= 276 kg Air16 kg Air LECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE18 Example 11-3. combustion of Methane (CH4)Solving for % excess air:Overall combustion reaction for the CH4:CH4+ 2O2========>CO2+ 2H2 OFor 16 kg of C3H8, the theoretical air required is:424821kmol CH2 O1 Air29 kg Air16 kg CH4= 276 kg Air16 kg Air300 kg Air - 276 kg Air% excess air = 100 = kg Air LECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE19 Example 11-4.
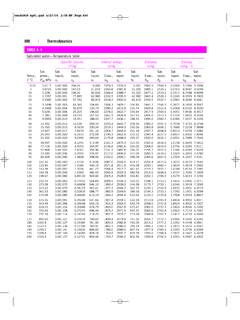
9 combustion of CoalA local utility burns coal having the following composition on a dry 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE20 Example 11-4. combustion of CoalThe average Orsatanalysis of the flue gas during a 24-hr test was:ComponentPercentCO2+ 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE21 Example 11-4. combustion of CoalMoisture in the fuel was and the air on the average contained lbm H2O/lbm dry air. The refuse showed combined elements as in the coal ( C, H, O, N, S) and the remainder being ash. It may be assumed that these combined elements occur in the same proportions as they do in the the amount of amount of flue-gas (dry basis), amount of water coming out of the process, and the %excess 10.
10 Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE22 Example 11-4. combustion of CoalLECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE23 Example 11-4. combustion of CoalBasis: 100 lbm of coalAsh (100 lbm) = = lbmCombustible elements in refuse ( lbm) = lbmAssuming the combustible elements (C, H, O, N, S) occur in the same proportions as they do in the coal, the quantities of the combustibles in R on an ash-free basis are:LECTURE 10. Solving Material Balance Problems Involving Reactive ProcessesProf. Manolito E Bambase Jr. Department of CHEMICAL ENGINEERING . University of the Philippines Los Ba os SLIDE24 Example 11-4. combustion of CoalComponentmass (lbm)ash-freemass %Amt. in R(lbm) R(lbmol) 10. Solving Material Balance Problems Involving Reactive ProcessesProf.