Transcription of Concentration and ideal gas formulas - VaxaSoftware
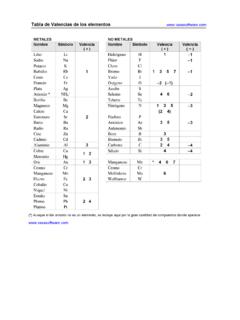
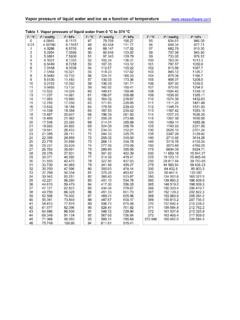
1 Concentration and ideal gas formulae solution litressolute moles MNormality solution litressolute sequivalent-gram N, Normality = Molarity ValencyMolality solvent kgsolute moles mMass-volume ratio solution litressolute grams/ LgMole fraction of solutesolvent moles+solute molessolute moles s Mass percentage 100solution masssolute mass% Volume-volume percentage 100solution volumesolute volume% Other formulae massmolar grams moles, mass equivalentgrams- sequivalentgvalencymassmolar mass Equivalent ; vmd=VolumeMassDensity ; ideal gas formulae P V=n R T 222111 TVPTVP TMmVP R TdMP R Where: P = Pressure (atm)V = Gas volume (L) n = Moles of gas (mol) T = Absolute temperature (K) R = atm L mol-1 K-1 Molar gas constant m = Mass of gas (g) M = Molar mass (g/mol) d = Density (g/L)Unit conversions1 atm = 760 mmHg = 760 torr = 101 325 Pa = 101 325 N/m2[K] = [ C] + : Standard temperature and pressure: 100 kPa, 0 C = K 1 mole of ideal gas has a volume of L at