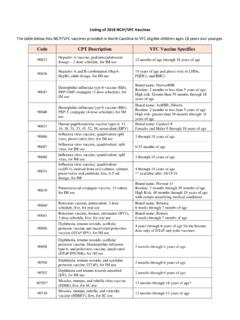
Transcription of CVX Code CVX Short Description Full Vaccine Name Note ...
1 CVX CodeCVX Short DescriptionFull Vaccine NameNoteVaccineStatusinternalIDnonvaccin eupdate_date54adenovirus, type 4adenovirus Vaccine , type 4, live, oralInactive1 False28-May-1055adenovirus, type 7adenovirus Vaccine , type 7, live, oralInactive2 False28-May-1082adenovirus, unspecified formulationadenovirus Vaccine , unspecified formulationThis CVX code allows reporting of a vaccination when formulation is unknown (for example, when r Inactive3 False30-Sep-1024anthraxanthrax vaccineActive4 False11-Jun-1919 BCGB acillus Calmette-Guerin vaccineActive5 False28-May-1027botulinum antitoxinbotulinum antitoxinActive6 True4-Sep-2026cholera, unspecified formulationcholera Vaccine , unspecified formulationInactive7 False17-Jun-1629 CMVIG cytomegalovirus immune globulin, intravenousActive8 True4-Sep-2056dengue feverdengue fever vaccineApplies to tetravalent product ( DENGVAXIA)Active9 False19-May-2212diphtheria antitoxindiphtheria antitoxinActive10 True4-Sep-2028DT (pediatric))
2 Diphtheria and tetanus toxoids, adsorbed for pediatric useActive11 False28-May-1020 DTaPdiphtheria, tetanus toxoids and acellular pertussis vaccineActive12 False28-May-10106 DTaP, 5 pertussis antigensdiphtheria, tetanus toxoids and acellular pertussis Vaccine , 5 pertussis antigensActive13 False28-May-10107 DTaP, unspecified formulationdiphtheria, tetanus toxoids and acellular pertussis Vaccine , unspecified formulationThis CVX code allows reporting of a vaccination when formulation is unknown (for example, when r Inactive14 False30-Sep-10110 DTaP-Hep B-IPVDTaP-hepatitis B and poliovirus vaccineActive15 False28-May-1050 DTaP-HibDTaP-Haemophilus influenzae type b conjugate vaccineInactive16 False20-Mar-17120 DTaP-Hib-IPVdiphtheria, tetanus toxoids and acellular pertussis Vaccine , Haemophilus influenzae type b con Active17 False28-May-10130 DTaP-IPVD iphtheria, tetanus toxoids and acellular pertussis Vaccine , and poliovirus Vaccine , inactivatedActive19 False28-May-1001 DTPdiphtheria.)
3 Tetanus toxoids and pertussis vaccineInactive21 False28-May-1022 DTP-HibDTP-Haemophilus influenzae type b conjugate vaccineInactive22 False23-Mar-17102 DTP-Hib-Hep BDTP- Haemophilus influenzae type b conjugate and hepatitis b vaccineThis CVX has been corrected. This non-US Vaccine contained DTP, Hib and Hep B. It was withdrawn Non-US23 False20-May-1657hantavirushantavirus vaccineNever Active24 False9-Sep-2052 Hep A, adulthepatitis A Vaccine , adult dosageActive25 False28-May-1083 Hep A, ped/adol, 2 dosehepatitis A Vaccine , pediatric/adolescent dosage, 2 dose scheduleActive26 False28-May-1084 Hep A, ped/adol, 3 dosehepatitis A Vaccine , pediatric/adolescent dosage, 3 dose scheduleThis Vaccine formulation is inactive and should not be used, except to record historic vaccinations w Inactive27 False28-May-1031 Hep A, pediatric, unspecified formulationhepatitis A Vaccine , pediatric dosage, unspecified formulationDo NOT use this code.
4 If formulation is unknown, use CVX 85. There is only one formulation of Hep Inactive28 False30-Sep-1085 Hep A, unspecified formulationhepatitis A Vaccine , unspecified formulationThis CVX code allows reporting of a vaccination when formulation is unknown (for example, when r Inactive29 False30-Sep-10104 Hep A-Hep Bhepatitis A and hepatitis B vaccineActive30 False28-May-1030 HBIG hepatitis B immune globulinActive31 True4-Sep-2008 Hep B, adolescent or pediatrichepatitis B Vaccine , pediatric or pediatric/adolescent dosageThis code applies to any standard pediatric formulation of Hepatitis B Vaccine . It should not be used Active32 False10-May-1842 Hep B, adolescent/high risk infanthepatitis B Vaccine , adolescent/high risk infant dosageAs of August 27, 1998, Merck ceased distribution of their adolescent/high risk infant hepatitis B vac Inactive33 False28-May-1043 Hep B, adulthepatitis B Vaccine , adult dosageAs of September 1999, a 2-dose hepatitis B schedule for adolescents (11-15 year olds) was FDA app Active34 False10-May-1844 Hep B, dialysishepatitis B Vaccine , dialysis patient dosageNDCs that are used for both Vaccine and dialysis ( Engerix-B))
5 Are mapped to CVX 43 which is not Active35 False8-May-1945 Hep B, unspecified formulationhepatitis B Vaccine , unspecified formulationThis CVX code allows reporting of a vaccination when formulation is unknown (for example, when r Inactive36 False30-Sep-1058 Hep Chepatitis C vaccineNever Active37 False9-Sep-2059 Hep Ehepatitis E vaccineNever Active38 False9-Sep-2060herpes simplex 2herpes simplex virus, type 2 vaccineNever Active39 False9-Sep-2046 Hib (PRP-D)Haemophilus influenzae type b Vaccine , PRP-D conjugateInactive40 False28-May-1047 Hib (HbOC)Haemophilus influenzae type b Vaccine , HbOC conjugateInactive41 False28-May-1048 Hib (PRP-T)Haemophilus influenzae type b Vaccine , PRP-T conjugateActive42 False28-May-1049 Hib (PRP-OMP))
6 Haemophilus influenzae type b Vaccine , PRP-OMP conjugateActive43 False28-May-1017 Hib, unspecified formulationHaemophilus influenzae type b Vaccine , conjugate unspecified formulationInactive44 False30-Sep-1051 Hib-Hep BHaemophilus influenzae type b conjugate and Hepatitis B vaccineThis Vaccine may still be in use until the existing lots expire on 8/19 immunodeficiency virus vaccineNever Active46 False9-Sep-20118 HPV, bivalenthuman papilloma virus Vaccine , bivalentInactive47 False28-May-1062 HPV, quadrivalenthuman papilloma virus Vaccine , quadrivalentActive49 False2-Jun-2086 IGimmune globulin, intramuscularActive51 True4-Sep-2087 IGIV immune globulin, intravenousActive52 True4-Sep-2014IG, unspecified formulationimmune globulin, unspecified formulationInactive53 True4-Sep-20111influenza, live, intranasalinfluenza virus Vaccine , live, attenuated, for intranasal useLAIV3 Inactive54 False22-Sep-1415influenza, split (incl.)
7 Purified surface antigen)influenza virus Vaccine , split virus (incl. purified surface antigen)-retired CODEThis code is being retired. It will still be found in older immunization records. It included both prese Inactive55 False30-Sep-1016influenza, wholeinfluenza virus Vaccine , whole virusInactive56 False28-May-1088influenza, unspecified formulationinfluenza virus Vaccine , unspecified formulationThis CVX code allows reporting of a vaccination when formulation is unknown (for example, when r Inactive57 False30-Sep-10123influenza, H5N1-1203influenza virus Vaccine , H5N1, A/Vietnam/1203/2004 (national stockpile)Inactive58 False28-May-1010 IPVpoliovirus Vaccine , inactivatedActive60 False28-May-1002 OPVtrivalent poliovirus Vaccine , live, oralThis is the OPV that was used in the USInactive61 False10-Feb-1789polio, unspecified formulationpoliovirus Vaccine , unspecified formulationThis CVX code allows reporting of a vaccination when formulation is unknown (for example.)
8 When r Inactive62 False30-Sep-1039 Japanese encephalitis SCJapanese Encephalitis Vaccine SCInactive63 False24-Sep-1563 Junin virusJunin virus vaccineNever Active64 False9-Sep-2064leishmaniasisleishmaniasi s vaccineNever Active65 False9-Sep-2065leprosyleprosy vaccineNever Active66 False9-Sep-2066 Lyme diseaseLyme disease vaccineInactive67 False28-May-1003 MMRmeasles, mumps and rubella virus vaccineActive68 False28-May-1004M/Rmeasles and rubella virus vaccineInactive69 False28-May-1094 MMRV measles, mumps, rubella, and varicella virus vaccineActive70 False28-May-1067malariamalaria vaccineNever Active71 False9-Sep-2005measlesmeasles virus vaccineInactive72 False31-Aug-1068melanomamelanoma vaccineNever Active73 False9-Sep-2032meningococcal MPSV4meningococcal polysaccharide Vaccine (MPSV4)Inactive74 False27-Oct-17103meningococcal C conjugatemeningococcal C conjugate vaccineInactive75 False28-May-10114meningococcal MCV4 Pmeningococcal polysaccharide (groups A, C, Y and W-135)
9 Diphtheria toxoid conjugate Vaccine Active76 False28-May-10108meningococcal ACWY, unspecified formulatiomeningococcal ACWY Vaccine , unspecified formulationThis CVX is intended for use when one of the meningococcal vaccines containing serogroups A, C, W Inactive77 False1-Dec-1507mumpsmumps virus vaccineInactive79 False18-Jun-1569parainfluenza-3parainflu enza-3 virus vaccineInactive80 False28-May-1011pertussispertussis vaccineInactive81 False28-May-1023plagueplague vaccineInactive82 False17-May-1833pneumococcal polysaccharide PPV23pneumococcal polysaccharide Vaccine , 23 valentActive83 False28-May-10100pneumococcal conjugate PCV 7pneumococcal conjugate Vaccine , 7 valentInactive84 False26-Jun-14109pneumococcal, unspecified formulationpneumococcal Vaccine , unspecified formulationThis CVX code allows reporting of a vaccination when formulation is unknown (for example, when r Inactive85 False30-Sep-1070Q feverQ fever vaccineNever Active86 False9-Sep-2018rabies, intramuscular injectionrabies Vaccine , for intramuscular injection RETIRED CODEThis CVX code has been retired.)
10 It is replace by CVX 175, or CVX 176. It will continue to be found in Inactive87 False18-Oct-1640rabies, intradermal injectionrabies Vaccine , for intradermal injectionInactive88 False18-Jun-1590rabies, unspecified formulationrabies Vaccine , unspecified formulationThis CVX code allows reporting of a vaccination when formulation is unknown (for example, when r Inactive89 False30-Sep-1072rheumatic feverrheumatic fever vaccineNever Active90 False9-Sep-2073 Rift Valley feverRift Valley fever vaccineNever Active91 False9-Sep-2034 RIGrabies immune globulinActive92 True4-Sep-20119rotavirus, monovalentrotavirus, live, monovalent vaccineActive93 False28-May-10122rotavirus.)