Transcription of Effect of Alloying Elements on Hydrogen Pickup in ...
1 Adrien Couet,1 Arthur T. Motta,2and Robert J. Comstock3 Effect of Alloying Elements onHydrogen Pickup in ZirconiumAlloysReferenceCouet, Adrien, Motta, Arthur T., and Comstock, Robert J., Effect of Alloying Elements onHydrogen Pickup in Zirconium Alloys, Zirconium in the Nuclear Industry: 17th InternationalSymposium, STP 1543, Robert Comstock and Pierre Barberis, Eds., pp. 479 509, , ASTM International, West Conshohocken, PA the optimization of zirconium-based alloys has led to significantimprovements in Hydrogen Pickup and corrosion resistance, the mechanisms bywhich such alloy improvements occur are still not well understood. In an effort tounderstand such mechanisms, we conducted a systematic study of the alloyeffect on Hydrogen Pickup , using advanced characterization techniques torationalize precise measurements of Hydrogen Pickup .
2 The Hydrogen pickupfraction was accurately measured for a specially designed set of commercial andmodel alloys to investigate the effects of Alloying Elements , microstructure, andcorrosion kinetics on Hydrogen uptake. Two different techniques for measuringhydrogen concentrations were used: a destructive technique, vacuum hotextraction, and a non-destructive one, cold neutron prompt gamma activationanalysis. The results indicate that Hydrogen Pickup varies not only from alloy toalloy, but also during the corrosion process for a given alloy. These variationsresult from the process of charge balance during the corrosion reaction, suchthat the Pickup of Hydrogen decreases when the rate of electron transport orManuscript received December 25, 2012; accepted for publication June 26, 2013; published online June 17, of Mechanical and Nuclear Engineering, Penn State Univ.
3 , University Park, PA 16802, United States ofAmerica (Corresponding author), e-mail: of Mechanical and Nuclear Engineering, Penn State Univ., University Park, PA 16802, United States Electric Company LLC, Pittsburgh, PA 15235, United States of 17th International Symposium onZirconium in the Nuclear Industryon February 3 7, 2013 inHyderabad, by ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA IN THE NUCLEAR INDUSTRY:17TH INTERNATIONAL SYMPOSIUM479 STP 1543, 2014 / available online at / doi: electronic conductivity (roxe) through the protective oxide to this hypothesis, Alloying Elements (either in solid solution or inprecipitates) would affect the Hydrogen Pickup fraction by mechanism whereby these Alloying Elements are incorporated into the oxidelayer is critical to changing electron conductivity, the evolution of the oxidation stateof two common Alloying Elements , Fe and Nb, when incorporated into the growingoxide layers of two commercial zirconium alloys (Zircaloy-4 and ZIRLO) and modelalloys ( and )
4 Was investigated using x-ray absorption near-edge spectroscopy with microbeam synchrotron radiation on cross-sectional oxidesamples. The results showthat the oxidation of both Fe and Nb is delayed in theoxide layer relative to that of Zr, and that this oxidation delay is related to thevariations of the instantaneous Hydrogen Pickup fraction with exposure pick-up, Zirconium alloys, CNPGAA, XANES, Alloying Elements , elec-tronic conductivityIntroductionAs fuel burnup and reactor residence times increase, the uniform corrosion of zir-conium alloy nuclear fuel cladding (and associated Hydrogen Pickup ) can become alimiting factor for the use of high-burnup fuel rods in existing and advanced light waterreactors [1,2].
5 Several factors can control the uniform corrosion of zirconium alloys [3].Although alloy optimization of zirconium-based alloys used for nuclear fuel claddinghas been a key to increasing corrosion resistance and reducing Hydrogen Pickup , acomplete understanding of the role of alloyingelementsinthecorrosionandhydroge npickup mechanisms is still very small Alloying element differences cause significant differences incorrosion and Hydrogen Pickup , it is of interest to examine the Effect of Alloying ele-ments on the corrosion and Hydrogen Pickup mechanisms of zirconium alloys,which can, in turn, yield significant insights potentially leading to validation of ageneral hypothesis on the underlying overall zirconium corrosion reaction is written asZr 2H2O!
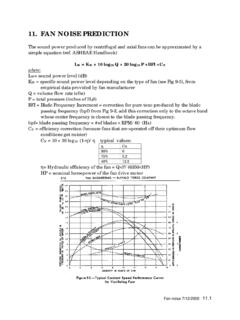
6 ZrO2 2H2(1)Some of the Hydrogen generated by the corrosion reaction diffuses through the ox-ide and enters the metal, where it eventually can precipitate as hydrides, causingcladding embrittlement [1]. For comparison between alloys, the total hydrogenpickup fractionfHis defined as the ratio of the Hydrogen absorbed from the begin-ning of the corrosion test,Dt0 Habsorbed, to the total amount of Hydrogen that hasbeen generated by the corrosion reaction, Dt0 HabsorbedDt0 Hgenerated(2)480 STP 1543On Zirconium in the Nuclear IndustryThe instantaneous Hydrogen Pickup fractionfiHis defined as the ratio of the hydro-gen absorbed from a timetto a timet Dtto the total amount of Hydrogen thathas been generated by the corrosion reaction during the same Dt DttHabsorbedDt DttHgenerated(3)
7 Few measurements of instantaneous Hydrogen Pickup fractions have beenreported in the literature, as they require precise Hydrogen measurements at suc-cessive small exposure time intervals. The determination offH(and, to a lesserextent,fiH) on various zirconium alloys has been the subject of extensiveresearch, but the mechanisms of Hydrogen Pickup and, especially, the influenceof the alloy composition and microstructure onfHare still not well understood[4 7]. Studies have shown thatfHdepends strongly on alloy composition [4,6],alloy microstructure [8,9], and corrosion conditions [10]. It has been shown inthe protective regime that pure Zr picks up about 20 % to 30 % of the hydrogengenerated during the corrosion reaction, more than commercial alloys [11].
8 Ingeneral, Alloying Elements decrease Hydrogen Pickup , with the exceptions of Ni,which increasesfH, and Sn, which is reported to have no Effect [11,12]. Previousmeasurements of instantaneous Hydrogen Pickup have shown thatfiHmay varyat different stages of oxide film growth [13], but no clear understanding yetexists. Some of the literature is reviewed in the following and Hydrogen Pickup MechanismsRATE-LIMITING STEP IN UNIFORM ZIRCONIUM ALLOY CORROSIONThe zirconium oxide formed on Zr alloys exhibits a protective character, such thatafter the formation of the oxide there is no direct contact between the metal andthe water, and the corrosion reaction cannot happen directly, so the oxidizing spe-cies have to travel through the oxide layer.
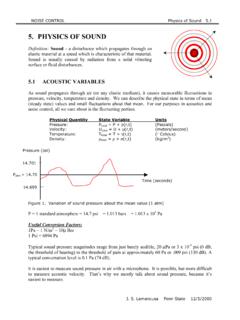
9 In order for zirconium oxidation tooccur, either the cations or the anions have to be transported through the oxidelayer. The corrosion of zirconium and its alloys in high-temperature environmentsoccurs via oxygen anion migration through the corrosion film, with the formationof new oxide occurring at the metal oxide interface [14,15]. The sub-stoichiometric gradient of the zirconium oxide would be the primary driving forcefor the oxygen anion diffusion, although this value has not been oxidation process can be divided into several steps, as presented inFig. , oxygen in the water molecule dissociates and is adsorbed onto the oxide !dissociation4H 2O2 adsorbed(4)COUET ET AL.
10 , DOI adsorbed V O !absorptionO2 absorbed(5)Because of the defect concentration gradient, the oxygen anions diffuse either the oxide metal interface, it reacts with Zr cations to form new !oxidationZr4 4e (6)Zr4 2O2 absorbed !oxideformationZrO2(7)The formation of this new oxide releases electrons, which then migrate through theoxide to reduce the Hydrogen ions at the cathodic 4e !reduction2H2(8)It is well known that the corrosion rate of zirconium alloys decreases as the thick-ness of the oxide layer increases [16]. Because of this, it is considered that either ox-ygen anion diffusion or electron diffusion is the rate-limiting step. This assumptionleads to the parabolic scaling law for the oxidation kinetics:d Ktn, withn [17].