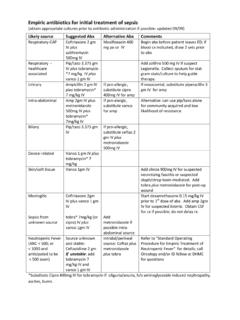
Transcription of EMPIRIC TREATMENT OF FEBRILE NEUTROPENIA
1 EMPIRIC TREATMENT OF FEBRILE NEUTROPENIA Disclaimer Both the format and content of the guidelines will change as they are reviewed and revised on a periodic basis. Any physician using these guidelines to provide TREATMENT for patients will be solely responsible for verifying the doses, providing the prescriptions, and administering the medications described in the guidelines, according to acceptable standards of care. Additional notes: Empirical ANTIFUNGAL therapy should be considered in patients, who are experiencing persistent fevers, despite receiving 3-5 days of broad-spectrum antibiotic therapy.
2 METRONIDAZOLE may be added to empirical IV antibiotics, if anaerobic infection ( , intra-abdominal) is suspected. Antimicrobial therapy should be continued until the infection has resolved and the patient is no longer neutropenic. In the absence of serious infections, G-CSF is not indicated to improve clinical outcomes, but may reduce hospitalization by 1 day. ADULT FEBRILE NEUTROPENIC PATIENT ANC < x 109/L (& expected to further decline) AND ORAL TEMPERATURE OC OR OC for 1 hour LOW RISK May treat as outpatient Features: Absolute neutrophil count > x 109 /L Absolute monocyte count > x 109 /L Normal findings on a chest radiograph Nearly normal liver and renal function tests Duration of NEUTROPENIA 7 days Resolution of NEUTROPENIA expected in < 10 days No intravenous catheter site infection Early evidence of bone marrow recovery Malignancy in remission Peak temperature of < oC No neurological or mental changes No abdominal pain, or appearance of illness No comorbid complications, , shock, hypoxia, pneumonia, serious infection, etc.
3 INTERMEDIATE RISK (Neither low nor high risk) Consider admitting patient ADDITIONAL CRITERIA: Reliable PATIENT, who can return to the facility easily Can take oral medications and fluids Can be easily contacted for daily assessment Can be admitted urgently, if clinically unwell/unstable HIGH RISK Admit Features: Age > 70 years Inpatient status at time of fever Significant medical comorbidity or clinically unstable, , hypotension, COPD, hypoxia, new onset abdominal pain, neurological changes, dehydration, etc. Anticipated prolonged severe NEUTROPENIA : ANC x 109 /L for > 7 days Serum creatinine > 176 mol/L Liver function tests > 3 x upper normal limit Uncontrolled, progressive cancer Pneumonia or other complex infections Mucositis grade > 2 Poor performance status (ECOG > 1) Intravenous catheter site infection FORMALLY RE-EVALUATE PATIENT IN 2 to 3 DAYS.
4 IF AFEBRILE for > 48 HOURS, AND NEUTROPHILS > X 10 9/ L for 2 consecutive days and increasing, no positive source of infection identified and patient clinically stable, may discontinue antibiotics and monitor patient. IF FEBRILE , admit patient for further investigations and initiation of appropriate antimicrobial therapy. RECOMMENDED ANTIBIOTICS: (Please check local hospital FORMULARY) (Hotlink to recommended doses) Intravenous PIPERACILLIN-TAZOBACTAM, OR Intravenous IMIPENEM OR MEROPENEM, OR Intravenous CEFEPIME OR CEFTAZIDIME (NOT recommended as monotherapy in areas at risk for extended-spectrum beta-lactamases [ESBL] producing bacteria) Intravenous AMINOGLYCOSIDE ( , Tobramycin / Gentamicin) OR CIPROFLOXACIN may be added to the initial EMPIRIC antibiotic regimen, if resistance is suspected or if there are complications ( , hypotension, persistent fever, pneumonia, etc.)
5 Intravenous VANCOMYCIN may be added, in the following situations: hemodynamic instability or sepsis, pneumonia, positive blood culture for gram-positive organism, catheter-related infection, skin or soft tissue infection, known or suspected MRSA, severe mucositis while receiving fluoroquinolone prophylaxis. Stop Vancomycin in 48 hrs, if not indicated. If anaphylaxis allergy to beta-lactams, treat with VANCOMYCIN + AMINOGLYCOSIDE + CIPROFLOXACIN. IF POSSIBLE, AVOID AMINOGLYCOSIDES OR OTHER NEPHROTOXIC AGENTS IN PATIENTS, RECEIVING CISPLATIN OR OTHER NEPHROTOXIC CHEMOTHERAPY. These guidelines are compiled from the published literature and current practice (Hotlink to references). For more information, please contact Dr. Shirin Abadi at To contact individual BCCA Centres, please call: Abbotsford (AC): 604-851-4710, Kelowna (CSI): 250-712-3900, Prince George (CN): 250-645-7300, Surrey (FVC): 604-930-2098, Vancouver (VC): 604-877-6000, Victoria (VIC): 250-519-5500.
6 OUTPATIENT THERAPY INPATIENT RECOMMENDED ANTIBIOTICS: (Hotlink to recommended doses) ORAL CIPROFLOXACIN + ORAL AMOXICILLIN/CLAVULANATE If anaphylaxis allergy to beta-lactams, consider ORAL CIPROFLOXACIN + ORAL CLINDAMYCIN CIPROFLOXACIN not recommended, if significant patient exposure in the past 3 months Not recommended for children see guidelines OTHERS ADMIT (See Recommended Antibiotics under HIGH RISK section) EMPIRIC TREATMENT OF FEBRILE NEUTROPENIA Disclaimer Both the format and content of the guidelines will change as they are reviewed and revised on a periodic basis. Any physician using these guidelines to provide TREATMENT for patients will be solely responsible for verifying the doses, providing the prescriptions, and administering the medications described in the guidelines, according to acceptable standards of care.
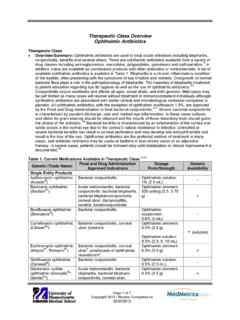
7 SUGGESTED DOSING FOR ANTIBIOTICS (IN ADULT PATIENTS WITH NORMAL RENAL FUNCTION): Amoxicillin/Clavulanate PO 500/125 mg Q8H, OR 875/125 mg Q12H Cefepime Ceftazidime IV IV 2 g Q8H 2 g Q8H Ciprofloxacin IV 400 mg Q8-12H PO 750 mg Q12H Clindamycin Gentamicin OR Tobramycin PO IV 600 mg Q8H 6-7 mg/kg Q24H (if CrCl > 60 mL/minute, otherwise use caution & prolong dosing interval) Imipenem IV 500 mg Q6H Meropenem IV 1 g Q8H Piperacillin/Tazobactam Ticarcillin/Clavulanate Vancomycin IV IV IV g Q6H g Q4-6H 25 mg/kg IV loading dose, followed by 15 mg/kg Q12H (round to nearest 250 mg dose) Metronidazole IV 500 mg Q12H References: 1. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America.
8 Clin Infect Dis 2011;52(4):e56-e93. 2. Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and outpatient management of fever and NEUTROPENIA in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013;31(6):794-810. 3. in:DN Gilbert, RC Moellering Jr, GM Eliopoulos, HF Chambers, MS Saag (Eds.). The Sanford Guide to Antimicrobial Therapy 2013. 43rd ed. Antimicrobial Therapy, Inc. Sperryville, VA; 2013. 4. National Comprehensive Cancer Network (NCCN). (2013). Prevention and TREATMENT of Cancer-Related Infections Retrieved May 26th, 2014, from 5. Bow E, Wingard JR. Overview of neutropenic fever syndromes. In: UpToDate, Marr KA , Thorner AR (Eds), UpToDate, Waltham, MA. (Accessed on May 26th, 2014). 6. Klastersky J, Paesmans M, Rubenstein EB, et al.
9 The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk FEBRILE neutropenic cancer patients. J Clin Oncol 2000;18(16):3038-51. Approved on: March 26th, 2015