Transcription of Guidelines for the treatment of malaria in South Africa, 2009
1 Guidelines FOR THE. treatment OF malaria . IN. South AFRICA. 2009. i TABLE OF CONTENTS. Preface iii Acknowledgem ents iv Abbreviations v Summary vi treatment of malaria in South Africa - Summ ary Flowchart 2007 vii 1. Introduction 1. 2. Objectives 2. 3. Parasite species 2. 4. Risk groups 2. 5. Clinical presentation and diagnosis 3. Sym ptoms and s igns 3. Laboratory diagnosis 5. Referral criteria 6. 6. treatment 8. Uncomplicated Plasmodium falciparum m alaria 8. Chemotherapy 8. General m anagement 9. Drugs us ed in the treatment of Plasm odium falciparum m alaria 11. Artem is inin-bas ed com bination therapy 11. Quinine 12. Treatm ents not recomm ended for falciparum malaria 13. treatment of non-Plasm odium falciparum infections 14. treatment of mixed Plasmodium s pecies infections 15. treatment during pregnancy 15. Diagnos is of m alaria in pregnancy 16. Managem ent of uncomplicated malaria in pregnancy 16. Managem ent of severe malaria in pregnancy 17.
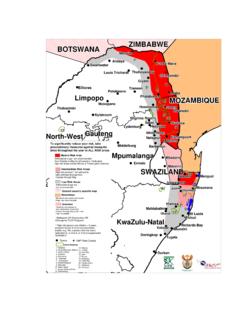
2 Treatm ent of infants and young children 18. Managing uncom plicated (non-severe) m alaria in young children 18. Managing severe malaria in young children 19. malaria and HIV 20. ii 7. Severe malaria 22. Features indicating severe m alaria 22. Treatm ent 24. Chemotherapy 24. Loading doses 25. Maintenance dos es 25. Other chemotherapeutic options 26. General m anagement pf severe m alaria 26. Complications 28. Anaem ia 28. Hypoglycaemia 28. Cerebral m alaria 29. Renal failure 30. Circulatory collapse 30. Metabolic acidos is 31. Res piratory com plications 32. Hepatic dysfunction 33. Diss em inated intravas cular coagulation (DIC) 33. Secondary infections 33. Hyperparasitaem ia 34. Malarial haem oglobinuria 35. Exchange trans fusion 35. Splenic rupture 35. 8. References 37. 9. Dosage Guidelines for the treatment of malaria 42. 10. Summary of malaria treatment Guidelines 45. 11. malaria risk map for South Africa 47. iii PREFACE.
3 Cons iderable s uccess has been achieved in the control and m anagement of malaria in South Africa in recent years . This is des pite the ongoing development of paras ite and vector resis tance to drugs and insecticides , res pectively. It gives me great pleas ure to introduce these Guidelines on the treatm ent of m alaria in South Africa. The objectives of thes e publications are to provide all those involved in the m anagement of m alaria with clear and practical Guidelines for the diagnosis and appropriate treatment of m alaria. The intended treatment outcomes are the prevention of m alaria morbidity and mortality. In addition the recommendations are intended to contribute to a reduced malaria transmiss ion and to lim it res is tance to anti-m alarial drugs . These Guidelines are bas ed on the World health Organization's Guidelines for the treatment of m alaria. Additional literature surveys have been undertaken. Factors that were cons id ered in the choice of therapeutic options included: effectiveness, s afety, and im pact on malaria transmiss ion and on the emergence and s pread of anti-m alarial res is tance.
4 The previous Guidelines were compiled in Augus t 2002. Advances in the availability of anti- m alarial drugs and changes in res pect of transm iss ion, intens ity, drug resis tance and health care delivery in South Africa have prom pted revis ion of thes e Guidelines . It is hoped thes e Guidelines will facilitate effective, appropriate and timeous treatm ent of m alaria, thereby reducing the burden of this diseas e in our communities and in South Africa. --------------------------------------- Ms B Hogan, MP. Minis ter of health Date: iv ACKNOWLEDGEMENTS. The National Departm ent of health (DOH) and the National malaria Advis ory Group (MAG). initiated these revised treatment Guidelines . Without the contribution of the key pers ons m entioned below, thes e updated Guidelines would not have become a reality. Thes e pers ons include: Dr Lucille Blum berg, National Ins titute for Communicable Dis eases, National health Laboratory Services; Prof Karen Barnes, Departm ent of Clinical Pharm acology, University of Cape Town, Dr Cornelia Duvenage, Departm ent of Internal Medicine, 1 Military Hos pital.
5 Dr Jan van den Ende (MAG Chairperson), Drs Martin and Sim Inc and Toga Laboratories ;. Dr Etienne Immelm an, department of health , KwaZulu Natal, Dr Gerhard Swart, department of health and Social Services , Mpum alanga Province, Dr Frank Hans ford, department of health (SCAT Chairpers on); Mrs Lee Baker, Medicines Inform ation Cons ultant, Am aye za Information Centre. DOH officials: Dr Bonnie Maloba, Communicable Diseas e Control (CDC), Mr Devanand Moonasar (CDC), Ms Tsakani Furum ele (CDC), Dr. Eunice Mis iani (CDC), Dr. Frew Bens on (CDC), Ms Helecine Zeeman (Pharm acy Programm es and Planning), Mrs Nerine Willers (CDC) and Ms Mary Anne Groepe (WHO, South Africa). Furthermore, I would like to express m y gratitude to those persons who reviewed the guidelin es . Thes e pers ons include: Associate Professor John Frean, National Ins titute for Communicable Dis eas es , National health Laboratory Services and Profess or Gary Maartens , Departm ent of Clinical Pharmacolo gy, Univers ity of Cape Town.
6 ---------------------------------------- ------------ Mr T Ms eleku Director-General: Departm ent of health v ABBREVIATIONS. ACT Artem isinin-based com bination therapy ARDS Acute res piratory distress syndrome CDC Communicable Diseas e Control CNS Central nervous s ystem CVP Central venous press ure DIC Diss eminated intravas cular coagulation KZN KwaZulu-Natal NSAIDS Non-s teroidal anti-inflamm atory drugs RDT Rapid diagnos tic tests SP Sulphadoxine-pyrimetham ine vi SUMMARY. Plasmodium falciparum accounts for the m ajority of m alaria cas es in Southern Africa and m ay be ass ociated with severe and fatal disease. Almos t all South Africans are non- immune, including res idents of seas onal m alaria transm ission areas, and are therefore at ris k for developing severe m alaria. The diagnosis and management of malaria is urgent. Delayed diagnosis and inappropriate treatment are associated with significantly increased morbidity and mortality.
7 Classically, malaria presents with fever, rigors, headache and body pains, but the clinical features are non-specific and may be confused with many other diseases, especially influenza. A definitive diagnosis should be made promptly by demonstrating the parasite on microscopy of a blood smear or by using a rapid malaria antigen test. The choice of anti-malarial agents is dependent on the severity of illness and the pattern of drug resistance of the parasite in the geographical area where malaria was acquired. In keeping with the WHO recommendations, using anti-malarial drugs in combination is essential, and artemisinin-based combinations are preferred. For uncomplicated malaria acquired in South Africa, artem ether-lumefantrine (Coartem ) or alternatively quinine plus either doxyc ycline or clindam ycin is recommended. High-level resis tance precludes the us e of chloroquine for falciparum m alaria. Sulfadoxine- pyrim ethamine (SP) is no longer recommended.
8 For s evere malaria , quinine (with the addition of doxyc ycline or clindam ycin) is currently recommended. Patients with s evere m alaria will require hospital adm iss ion. All patients with m alaria require careful clin ical and parasitological follow-up. The m ajor complications of m alaria include: cerebral malaria , hypoglycaemia, anaemia, renal failure, acute res piratory dis tress s yndrom e (ARDS) and m etabolic acidos is , and thes e carry high m ortality rates especia lly in children, pregnant woman and in those living with HIV and AIDS. These complications require specific m anagem ent. DIS CLAIM ER. This material is intended for us e by healthcare professionals . It has been com piled from inform ation currently available, and although the greates t care has been taken the Departm ent of health and its malaria Advis ory Group do not accept res pons ibility for errors or omiss ions . Readers are referred to the reference articles for further inform ation and should exercise their own professional judgem ent in confirm ing and interpreting the findings pres ented in the publication.
9 Thes e Guidelines were iss ued in 2008 by the National Departm ent of health , and replace all previous Guidelines . vii treatment of malaria in South Africa- Summary Flowchart 2006. Confirm Diagnosis Quinine plus eith er & assess sev erity dox y cy cline or clindamy cin*. Or OR. Uncomplicated P. falciparum malaria - mild sy mptoms Artemether-lumefantrine (Coartem ). - ambulant - normal mental function - v omiting not persis tent - no organ dy sfunction IV Quinine plus either dox y cy cline or clindamy cin Severe P. falciparum malaria (See definition pg 22). P. ovale or P. vivax: chloroquine plus primaquine P. malariae: Other Plasmodium s pecies** chloroquine Clinical foll ow up and repeat blood smear * Add clindamy cin or dox y cycline as soon as can be tolerated after quinine is started. Clindamycin is preferred in children <8 y ears and pregnant women. ** If uns ure of species, treat as for P. falciparum. If mixed infection of P.
10 Falciparum plus P. vivax / ovale, treat as for P. falciparum plus follow with primaquine viii malaria treatment Guidelines Final Draft 23 June 2009. 1. INTRODUCTION. The relentless development of drug resistance in malaria parasites (notably P. falciparum), has necessitated ongoing updates of chemoprophylaxis and treatment policies globally. In South Africa, chloroquine resistance was first demonstrated in KwaZulu-Natal (KZN), and later in Mpumalanga. This prompted a policy change from chloroquine to sulphadoxine-pyrimethamine (SP) as first line treatment for uncomplicated malaria in KZN in 1988 and in Mpumalanga and Limpopo provinces in 1997. The development of significant SP resistance in KZN led to a further policy change in 2001, with artemether-lumefantrine replacing SP as first line treatment for uncomplicated P. falciparum infections. Subsequently, in December 2004, Limpopo Province also replaced SP with artemether-lumefantrine.