Most biochemical reactions occur in an aqueous environment ...
• Biological systems use buffers to maintain pH. • Definition: A buffer is a solution that resists a significant change in pH upon addition of an acid or a base. • Chemically: A buffer is a mixture of a weak acid and its conjugate base • Example: Bicarbonate buffer is a mixture of carbonic acid
Tags:
Information
Domain:
Source:
Link to this page:
Please notify us if you found a problem with this document:
Documents from same domain
Chapter 3: Solutions of Homework Problems …
www.csun.edu3 – 1 Chapter 3: Solutions of Homework Problems Vectors in Physics 12. as drawn at Picture the Problem: The given vector components correspond to the vector r & right.
Solutions, Physics, Problem, Vector, Homework, Solutions of homework problems, Solutions of homework problems vectors in physics
CHAPTER Classification and Assessment of …
www.csun.eduCHAPTER Classification and Assessment of Abnormal Behavior CHAPTER OUTLINE HOW ARE ABNORMAL BEHAVIOR PATTERNS CLASSIFIED? 70–77 The DSM and Models of Abnormal Behavior STANDARDS OF ASSESSMENT 77–80 Reliability Validity Cognitive Assessment
Assessment, Chapter, Classification, Chapter classification and assessment of
Dissociative and Somatoform Disorders
www.csun.edu212 Chapter 7 In early versions of the DSM, dissociative and somatoform disorders were classified with the anxiety disorders under the general category of …
Disorders, Dissociative and somatoform disorders, Dissociative, Somatoform
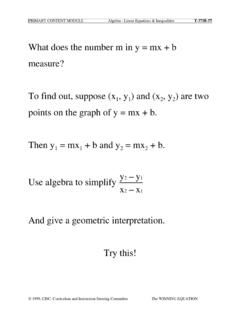
PRIMARY CONTENT MODULE Algebra - Linear …
www.csun.eduPRIMARY CONTENT MODULE Algebra - Linear Equations & Inequalities T-37/H-37 © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION
Linear, Primary, Content, Equations, Inequalities, Module, Primary content module algebra linear, Algebra, Primary content module algebra linear equations amp inequalities
Chapter 8: Quantitative Sampling
www.csun.edu22 Chapter 8: Quantitative Sampling I. Introduction to Sampling a. The primary goal of sampling is to get a representative sample, or a small collection of units
Chapter, Quantitative, Sampling, Chapter 8, Quantitative sampling
th - csun.edu
www.csun.eduRunning head: APA FORMAT EXAMPLE 1 How to Do that Annoying APA Format Stuff: A Brief Overview of the 6th Edition Scott W. Plunkett California State University, Northridge
Calculations and Occupational Exposure Limits
www.csun.eduCalculations Evaluation Control 3 5 OELs • Time-weighted average (TWA) • Ceiling value (C) • Short-Term Exposure Limit (STEL) • Immediately Dangerous to Life and Health
Health, Occupational, Calculation, Exposure, Occupational exposure
Employee Evaluation - California State University, …
www.csun.eduPerfEval (Rev. 04/2013) Page 3 Performance Categories Rating Ethics/Fraud/Integrity Practices excellent work ethics. Properly handles confidential information.
States, University, Evaluation, Employee, California, California state university, Employee evaluation
The Great Depression: California in the Thirties
www.csun.eduThe Great Depression: California in the Thirties . California was hit hard by the economic collapse of the 1930s. Businesses failed, workers lost …
California, Depression, Great, The great depression, California in the thirties, Thirties
Physics 100A Homework 4 – Chapter 5 Newton’s …
www.csun.eduChapter 5: Newton’s Laws of Motion thJames S. Walker, Physics, 4 Edition C) and D) Magnitude and direction of the acceleration
Related documents
pH buffers. Buffer capacity and buffering range
pg.edu.pl- buffer solution, pH = 4.01 - buffer solution, pH = 7.00 Mount the combined glass electrode in the holder. Thoroughly rinse the electrode with distilled water and gently dry with a piece of paper towel. Place the vial with buffer solution (pH 4.01) on the magnetic stirrer. Immerse the glass electrode in the buffer solution (the
Experiment 7: Preparation of a Buffer
jupiter.plymouth.eduSecond, you will make 100 mL of a buffer also with pH = 5, but with a higher buffering capacity, using 5 mL of a 0.5 M acetic acid solution. Although a buffer will resist a change in pH, eventually enough acid or base can be added to destroy it. The amount of acid or base needed to change the pH of a buffer is known as the "buffering capacity."
Solutions, Preparation, Experiment, Buffer, Experiment 7, Preparation of a buffer
0.1M Citric acid-Sodium citrate buffer buffer – pH range 3 ...
www.mystrica.com0.1M Citric acid-Sodium citrate buffer buffer – pH range 3.0 – 6.2 Prepare a 0.1M solution of citric acid monohydrate, C 6H8 O 7&H 2O (21.01g/l) and a 0.1M solution of trisodium citrate dihydrate, C 6H5 O 7Na 3&2H 2O (29.41g/l). Mix the volumes shown in the table. Or dissolve the masses shown and make up to 100cm 3 withwater
Applying Lime to Raise Soil pH for Crop Production ...
catalog.extension.oregonstate.eduBuffer—material that is resistant to pH change Slaked lime—calcium oxide that has been mixed with water, creating calcium hydroxide ... laboratory, very little H is in soil solution. Most of the H is exchangeable, or electrostatically attracted to the soil particles. The SMP buffer
Sample Exercise 17.1 Calculating the pH When a Common Ion ...
www.austincc.eduCalculating the pH of a Buffer. Calculate the pH of a buffer composed of 0.12 . M . benzoic acid and 0.20 . M . sodium benzoate. (Refer to Appendix D.) Answer: 4.42. Practice Exercise. Solution (Continued) Because K a is small and a common ion is present, we expect x to be small relative to either 0.12 or 0.10 M. Thus, our equation can be ...
pH of Seawater: The Role of Carbonate (CO3 and …
www.carboeurope.orgMc Crumb Indicator Solution* with colour chart pulverised eggshells (optional) Fig. 2 Materials *The McCrumb indicator solution has a pH range of 1-12. It turns from green to purple in basic solutions and from yellow to red in acidic solution. Procedure: 1. Fill three bottles with 200 ml seawater and the other 3 with 200 ml distilled water.
Solutions, Roles, Carbonates, Seawater, The role of carbonate, Co3 and
Protocol: Protein electrophoresis and western blot recipes
assets.thermofisher.com100 mM tricine, 0.1% SDS, pH 8.3 Recipe for 10X buffer stock: Tris base 121 g Tricine 179 g SDS 10 g Deionized water to 1,000 mL The buffer is stable for 6 months when stored at room temperature. Do not use acid or base to adjust pH. Bis-Tris transfer buffer: 25 mM bicine, 25 mM Bis-Tris (free base), 1 mM EDTA, pH 7.2 Recipe for 20X buffer stock:
Western, Bolt, Recipes, Protein, Buffer, Electrophoresis, Protein electrophoresis and western blot recipes
Potentiometry: The pH Electrode and Potentiometric …
www.edaq.comTwo point pH calibrations can be performed with any two standard pH buffers, however buffers of pH 4 and 9 (or 10) are commonly used. 6.2.1 Pour approximately 10 mL of either the Phthalate buffer or the Borax buffer into
Buffer, Electrode, Potentiometric, Ph electrode and potentiometric
Solutions for the problems about „Calculation of pH in the ...
www.chem.science.unideb.huWhat is the pH in a 0.010 M solution of a moderately weak acid if the Ka = 1.5 ... It will be a buffer system: pH = 4.952 13. A 10 mL sample of acetic acid with 0.1 M concentration is titrated with sodium hidroxide. The concentration of sodium hidroxide is 0.1 M as well. Decide whether it
Ion exchange chromatography - Thermo Fisher Scientific
tools.thermofisher.compH 7. A protein with a pI of 7 will bind more tightly to the cation column if its buffer is pH 3 rather than pH 4. The greater the electrostatic charge is, the more concentrated the sodium chloride must be to elute the target. An alternative to eluting by increasing the salt concentration is to appropriately alter the pH of the buffer. For ...