Transcription of Preferred reporting items for systematic review and meta ...
1 Preferredreportingitemsfor systematicreviewandmeta-analysisprotocol s(PRISMA-P)2015:elaborationand explanationLarissaShamseer1, DavidMoher1, MikeClarke2, DavinaGhersi3, AlessandroLiberati(deceased)4,MarkPettic rew5, Paul Shekelle6, LesleyA Stewart7, the PRISMA-PGroup1 OttawaHospitalResearchInstituteand Universityof Ottawa,Canada;2 Queen sUniversityBelfast,Ireland;3 NationalHealthand MedicalResearchCouncil,Australia;4 Universityof Modena,Italy;5 LondonSchoolof Hygieneand TropicalMedicine,UK;6 SouthernCaliforniaEvidence-basedPractice Center,USA;7 Centrefor Reviewsand Dissemination,Universityof York,UKDedication:The PRISMA-P2015initiativeis dedicatedto our colleagueAlessandroLiberati(1954 2012),who passedawaywhilePRISMA-P2015was underdevelopmentand whosecontributionsto this work systematicreviewsand meta-analysesallowfor planningand documentationof reviewmethods,act as a guardagainstarbitrarydecisionmakingdurin greviewconduct,enablereadersto assessforthe presenceof selectivereportingagainstcompletedreview s,and,whenmadepubliclyavailable,reducedu plicationof effortsand existenceof selectivereportingand excessiveduplicationof reviewson the sameor similartopicsis accumulatingand manycalls havebeenmadein supportofthe documentationand publicavailabilityof recentyearsto rectifytheseproblems,includingdevelopmen tof an internationalregisterfor prospectivereviews(PROSPERO)and launchof the first openaccessjournaldedicatedtothe exclusivepublicationof systematicreviewproducts,includingprotoc ols(BioMedCentral sSystematicReviews).
2 Furtheringtheseeffortsand buildingon the PRISMA(PreferredReportingItemsforSystema ticReviewsand Meta-analyses)guidelines,an internationalgroupof expertshas createda guidelineto improvethe transparency,accuracy,completeness,and frequencyof documentedsystematicreviewand meta-analysisprotocols PRISMA-P(for protocols) PRISMA-Pchecklistcontains17 itemsconsideredto be essentialand minimumcomponentsof a systematicreviewor PRISMA-P2015 Explanationand Elaborationpaperprovidesreaderswith a full understandingof and evidenceaboutthe necessityof eachitem as well as a modelexamplefrom an papershouldbe read togetherwith the assessorsare stronglyencouragedto makeuse of PRISMA-Pwhendraftingand a uniqueplacein helpform the basis for developingpracticeguidelinesand theyprovideinformationon gaps in knowledge,thus informationis relevantto stakeholdersacrossthe rigourand trustworthinessofsystematicreviewsis, in large part, basedon the a prioriplanningand documentationof a methodicalapproachtoconduct(that is, a protocol).
3 A systematicreviewprotocolis importantfor severalreasons:(1) it allowssystematicreviewersto plan carefullyand therebyanticipatepotentialproblems;(2) it allowsreviewersto explicitlydocumentwhat is plannedbeforethey start their review ,enablingothersto comparethe protocoland the completedreview(that is, to identifyselectivereporting),to replicatereviewmethodsif desired,and to judgethe validityof plannedmethods;(3) it preventsarbitrarydecisionmakingwith respectto inclusioncriteriaand extractionof data; and (4) it may reduceduplicationof effortsand enhancecollaboration, as the CochraneandCampbellCollaborationsand the Agencyfor HealthcareResearchand Quality(AHRQ)regularlyrequireand ,outsideof such organizations,few protocolsare publishedin traditionaljournalsand most reportsofcompletedreviews(89%)do not mentionworkingfrom aprotocol1(2014updateunderway).Manyexper tshave calledfor improveddocumentationand availabilityof response,experts(someof whomare authorson thisdocument)launchedan international,prospectiveregisterforsyst ematicreviewprotocols(PROSPERO, ) throughthe Centrefor Reviewsand Disseminationat the Universityof York (UK)in February2011,in whichmorethan 5000 systematicreviewprotocolsfrom 69 countrieshavebeen registeredas of February2012,theCorrespondence to: L Shamseer personal use only: See rights and reprints : ;349:g7647doi: (Published2 January2015)Page1 of 25 ResearchMethods& ReportingRESEARCHMETHODS& reporting first open accessjournalto exclusivelypublishsystematicreviewproduc tsincludingprotocols(BioMedCentral sSystematicReviews) was launched,in which142 protocolshave beenpublished(June2014).
4 Outsideof selectsystematicrevieworganizations,litt le to no generalguidanceexistsfor of the most importantfunctionsof systematicreviewprotocolsis their role as a documentationof plannedreviewmethods,outcomes,and analysesthat can be comparedwith completedreviewsto detectwhetherunintendedandundocumentedch angeswere relatedto selectivereportingof outcomes(that is, whenreportingis relatedto thestatisticalsignificanceor directionof effectestimate)is aproblemin is a well documentedphenomenonin clinicaltrials,2-7and similarfindingsare startingto emergefor systematicreviews(see item 13 for fulldiscussion).8-10 Whenreviewersselectivelychoosewhichinfor mationto includein a reportbasedon the directionandsignificanceof findings,they risk biasingthe evidencebase onwhichhealthcaredecisionsand policiesare recenteffortsto increasethe documentationandavailabilityof reviewprotocols,the next logicalstep is thedevelopmentof a set of standardsthat shouldbe includedin well describedprotocolmay facilitateandenhancethe detectionof undocumentedchangesto reviewmethodology.
5 It also may allowreadersto gaugethe potentialimpactof such changesas well as selectivereportingofinformationon that end, a reportingguidelinefor systematicreviewprotocols,an extensionof the PRISMA(PreferredItemsforReportingSystema ticReviewsand Meta-analyses)statementhas been developedfor protocols(PRISMA-P)and is describedin detailin this PRISMA-PPRISMA-Pis intendedto guidethe developmentof protocolsof systematicreviewsand systematicreviewsthat are not evaluatingefficacy,authorsare encouragedto use PRISMA-Pbecauseofthe lack of the purposeof this guidance,we definea protocol,broadly,as a documentwrittenbeforethe start of a systematicreviewdescribingtherationalean d intendedpurposeof the review ,and the plannedmethodologicaland analyticalapproach(see box 1 forcomprehensivedefinitions).PRISMA-Pis meantto be used primarilyby authorspreparingsystematicreviewprotocol sfor publication,publicconsumption,or is also intendedfor thosecommissioningandpotentiallyfundingr eviewsas a guidefor applicantson whatshouldthey shouldincludein their reviewprotocols,and as atool for peer reviewersto gaugewhethera also be helpfulfor journaleditorsand peer reviewersgaugingthe adequacyof reviewprotocolsfor list of stakeholdersto whomwebelievePRISMA-Pwill be usefulalongwith proposedbenefitsfor each groupis providedin table 1.
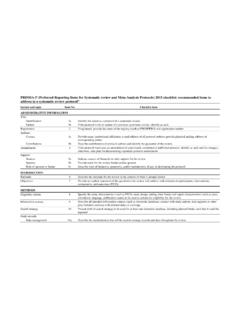
6 Developmentof PRISMA-PThe PRISMA-Pchecklistis basedon elementsfrom thePROSPERO register,11the PRISMA checklist,12 SPIRIT(StandardProtocolItems:Recommendat ionsfor InterventionalTrials)checklistitems,13an d from the InstituteofMedicine sStandardsfor detaileddescriptionof the steps undertakenduringPRISMA-Pdevelopmentcan be foundin the processfollowsgeneralrecommendationsof theEQUATOR(Enhancingthe Qualityand Transparencyof healthResearch)Networkon how to developa reportingguideline,ofwhichone fundamentalpart is a internationalexpertswas heldin June 2011 in Rockville,MD, USA,to developand relatedguidancedocumentshave undergoneiterativerevisionwithinthe PRISMA-PGrouplistedat the end of this document;membersof the PRISMA-PGroupcontributedto the writingand identifyingrelevantexamplesin this final PRISMA-Pchecklistcontains17 numbereditems(26sub- items )that shouldbe described,at minimum,in protocolsof systematicreviewsand meta-analyses(table2 ).
7 Thechecklistis dividedinto three main sections:administrativeinformation,intro duction,and observethat wordingof the PRISMA-Pchecklistshas, wherepossible,been harmonizedwith PRISMA checklistitems,at least 13 of whichare overlappingwith anticipatethis will aid authorsin transitioningtheirsystematicreviewprotoc olspreparedin accordancewithPRISMA-Pinto full text, PRISMA-compliant, ExplanationThe formatof this documentfollowsthat of previouslyestablishedreportingguidelines such as the PRISMAE xplanationand Elaborationdocument17; it aims to providereaderswith comprehensiveexplanationsand evidencebasedrationalesfor each good reportingfor each checklistitem have been identifiedfrom existingsystematicreviewand meta-analysisprotocolsand are providedthroughoutthis documentto a minimallist of itemstoconsiderwhenpreparinga systematicreviewprotocol,we haveindicatedinstanceswhereadditionalinf ormationmay bedesirableto improvetransparencyof the recommendationswithinPRISMA-Pmay requiremorewordsor spacethan authorsare accustomedto.
8 Providingdetaileddescriptionsfor someprotocolelements(suchas item8, eligibilitycriteria;item 13, outcomesand prioritisation)willfacilitatetransparenc yand futurereproducibility,and allowauthorsto shortentheir methodssectionin a completedsystematicreviewreport,if desired,by providinga briefsummaryof the methodsand referringreadersto the completedprotocolor believethat providingindepthdescriptionsof plannedmethodologicaldetailsforsystemati creviewsis in line with emergingjournalpoliciesaimedat numberedas we envisionthem appearingin a protocol,and reportingthem in this sequentialorderis asuggestionthat may orderof appearanceof checklistitemsif theydeemit to be importantis that authorsdescribeeach PRISMA-Pitem somewherein their personal use only: See rights and reprints : ;349:g7647doi: (Published2 January2015)Page2 of 25 RESEARCHMETHODS& REPORTINGBox 1: PRISMA-PterminologySystematicreview A systematicreviewattemptsto collateall relevantevidencethat fits pre-specifiedeligibilitycriteriato usesexplicit,systematicmethodsto minimizebias in the identification,selection,synthesis,and summaryof , this providesreliablefindingsfrom whichconclusionscan be drawnand 180 The keycharacteristicsof a systematicrevieware: (a) a clearlystatedset of objectiveswith an explicit,reproduciblemethodology;(b) a systematicsearchthat attemptsto identifyall studiesthat wouldmeetthe eligibilitycriteria;(c) an assessmentof the validityof the findingsof theincludedstudies(suchas assessmentof risk of bias and confidencein cumulativeestimates).
9 And (d) systematicpresentation,andsynthesis,of the characteristicsand findingsof the Meta-analysisis the use of statisticaltechniquesto combineand summarizethe resultsof multiplestudies;they mayor may not be containedwithina combiningdata from severalstudies,meta-analysescan providemorepreciseestimatesof the effectsof healthcarethan thosederivedfrom the In the contextof systematicreviewsand meta-analyses,a protocolis a documentthat presentsan explicitscientific roadmap of a planned, protocoldetailsthe rationaland plannedmethodologicalandanalyticalapproa chof the pointto note is that, whilethe developmentof a protocolabstractis not a listedrequirementon the PRISMA-Pchecklist,authorsare urgedto consultthe PRISMA extensionfor reportingconferenceand journalabstractsif so examplesand explanationsfor each checklistitem follow;citationscontainedwithinexamplesh ave been removedto avoidpotentialconfusionwith citationsin this : AdministrativeinformationTitleItem 1a: reportas aprotocolof a systematicreviewExample Postoperativeoutcomesfollowingpreoperati veinspiratorymuscletrainingin patientsundergoingopen cardiothoracicorupperabdominalsurgery:pr otocolfor a systematicreview 20 ExplanationThe knowledgein systematicreviewscan be harnessedonly ifreaderscan indicatethat systematicreviewsare not alwaysdescribedas such in eitherthe title orabstract.
10 Only 50% of systematicreviewsincludedin aNovember2004 sampleused the terms systematicreview or meta-analysis in their title or happens,reviewsandmeta-analysesmay not be indexedin databasesappropriatelyand risk not beingfoundby can lead towastedeffortsby systematicreviewerswhenknowledgetheyprod ucecannotbe identified,one consequenceof whichmaybe unnecessaryduplicationof effortsby their reportas a protocolof a systematicreviewand plannedmeta-analysis(the latter,only if knownatthe protocolstage).The term protocolindicatesthe existenceof a plan for an upcoming,ongoing,or a protocolmay reduceunnecessaryredundancyof systematicreviewefforts22and may also behelpfulfor readersseekingassistancein the designof beendevelopedto identifysystematicreviews,23inclusionof thetermssystematicreviewor, if a meta-analysisis planned,meta-analysisin the title of a protocolmay improveidentificationand adviseauthorsto use informativetitles that makekeyinformationeasilyaccessibleto ,a title reflectingthe PICO approach(participants,interventions,comp arators,and outcomes)as well as time frame,setting,and studydesign,if desired(see Item 7), will providereaderswith key informationaboutthe scopeof the 1b: the protocolis for an updateof a previoussystematicreview,identifyas suchExample The associationbetweenproximityto animal-feedingoperationsand communityhealth.