Transcription of Q7: What emissions from human activities lead to ozone ...
1 TWENTY QUESTIONS: 2006 human -produced chlorine and brominegases. human activities cause the emission of halogensource gasesthat contain chlorine and bromine emissions into the atmosphere ultimately lead tostratospheric ozone depletion. The source gases that con-tain only carbon, chlorine, and fluorine are called chlo -rofluorocarbons, usually abbreviated as CFCs. CFCs,along with carbon tetrachloride (CCl4) and methyl chlo -roform (CH3 CCl3), historically have been the most impor-tant chlorine-containing gases that are emitted by humanactivities and destroy stratospheric ozone (see Figure Q7-1).
2 These and other chlorine-containing gases have beenused in many applications, including refrigeration, air con-ditioning, foam blowing, aerosol propellants, and cleaningof metals and electronic components. These activitieshave typically caused the emission of halogen-containinggases to the category of halogen source gases containsbromine. The most important of these are the halons and methyl bromide (CH3Br). Halons are halogenatedhydrocarbon gases originally developed to extinguishfires. Halons are widely used to protect large computers,military hardware, and commercial aircraft of these uses, halons are often directly releasedinto the atmosphere.
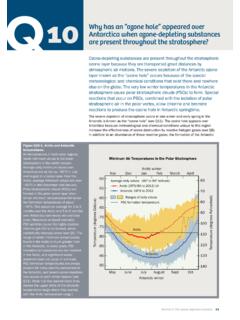
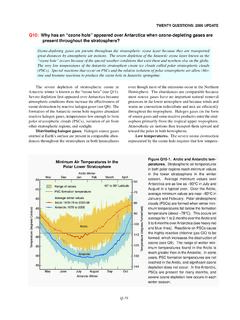
3 Halon-1211 and halon-1301 are themost abundant halons emitted by human activities (seeFigure Q7-1). Methyl bromide, used primarily as an agri-cultural fumigant, is also a significant source of bromineto the emissions of the principal chlorine- andbromine-containing gases have increased substantiallysince the middle of the 20thcentury (see Q16). The resulthas been global ozone depletion, with the greatest lossesoccurring in polar regions (see Q11 to Q13).Otherhuman sources of chlorine and chlorine- and bromine-containing gases are releasedregularly in human activities .
4 Common examples are theuse of chlorine gases to disinfect swimming pools andwastewater, fossil fuel burning, and various industrialprocesses. These activities do not contribute significantlyto stratospheric amounts of chlorine and bromine becauseeither the global source is small or the emitted gases areshort-lived (very reactive or highly soluble) and, there-fore, are removed from the atmosphere before they reachthe stratosphere. Natural sources of chlorine and a few halogen source gases present in the stratospherethat have large natural sources.
5 These include methylchloride (CH3Cl) and methyl bromide (CH3Br), both ofwhich are emitted by oceanic and terrestrial sources of these two gases contribute about 17%of the chlorine currently in the stratosphere and about 30%of the bromine (see Figure Q7-1). Very short-lived sourcegases containing bromine, such as bromoform (CHBr3),are also released to the atmosphere primarily from theoceans. Only a small fraction of these emissions reachesthe stratosphere, because these gases are rapidly removedin the lower atmosphere.
6 The contribution of these veryshort-lived gases to stratospheric bromine is estimated tobe about 24%, but this has a large con-tribution to stratospheric chlorine of short-lived chlori-nated gases from natural and human sources is muchsmaller (< 3%) and is included in the Other gases cat-egory in Figure Q7-1. Changes in the natural sources ofchlorine and bromine since the middle of the 20thcenturyare not the cause of observed ozone depletion. Lifetimes and emission, halogensource gases are either naturally removed from the atmos-phere or undergo chemical conversion.
7 The time toremove or convert about 60% of a gas is often called itsatmospheric lifetime. Lifetimes vary from less than 1year to 100 years for the principal chlorine- and bromine-containing gases (see Table Q7-1). Gases with the shortestlifetimes ( , the HCFCs, methyl bromide, methyl chlo -ride, and the very short-lived gases) are substantiallydestroyed in the troposphere, and therefore only a frac-tion of each emitted gas contributes to ozone depletion inthe stratosphere. The amount of a halogen source gas present in theatmosphere depends on the lifetime of the gas and theQ7:What emissions from human activities lead to ozone depletion?
8 Certain industrial processes and consumer products result in the emission of halogen source gases to theatmosphere. These gases bring chlorine and bromine to the stratosphere, which cause depletion of the ozonelayer. For example, chlorofluorocarbons (CFCs), once used in almost all refrigeration and air conditioningsystems, eventually reach the stratosphere, where they are broken apart to release ozone -depleting chlorineatoms. Other examples of human -produced ozone -depleting gases are the halons, which are used in fireextinguishers and contain ozone -depleting bromine atoms.
9 The production and consumption of all principalhalogen source gases by human activities are regulated worldwide under the Montreal QUESTIONS: 2006 emitted to the atmosphere. emissions vary greatlyfor the principal source gases, as indicated in Table of most gases regulated by the MontrealProtocol have decreased since 1990, and emissions fromall regulated gases are expected to decrease in the comingdecades (see Q16). ozone Depletion halogen sourcegases in Figure Q7-1 are also known as ozone -depletingsubstances because they are converted in the stratosphereto reactive gases containing chlorine and bromine (seeQ8).
10 Some of these reactive gases participate in reactionsthat destroy ozone (see Q9). ozone -depleting substancesare compared in their effectiveness to destroy strato-spheric ozone using the ozone Depletion Potential (ODP), as listed in Table Q7-1 (see Q18). Agas with alarger ODP has a greater potential to destroy ozone overits lifetime in the atmosphere. The ODP is calculated ona per mass basis for each gas relative to CFC-11, whichhas an ODP defined to be 1. Halon-1211 and halon-1301have ODPs significantly larger than CFC-11 and mostother emitted gases, because bromine is much more effec-tive overall (about 60 times) on a per-atom basis than chlo -rine in chemical reactions that destroy ozone in the strato-sphere.