Transcription of Zimmer Biomet Holdings, Inc. 1st Quarter 2018 Earnings ...
1 Zimmer Biomet Holdings, Inc. 1st Quarter 2018 Earnings Call Presentation April 26, 2018. Cautionary Note on Forward-Looking Statements and Non- GAAP Financial Measures Cautionary Note on Forward-Looking Statements. Our discussions during this presentation will include forward-looking statements concerning, among other things, our anticipated future operating and financial performance, business plans and prospects, product and service offerings, including new product launches and potential clinical successes, capital allocation plans and priorities, business development plans and priorities and the benefits expected from our acquisitions and other business development activities.
2 Such statements are based upon the current beliefs and expectations of management and are subject to significant risks, uncertainties and changes in circumstances that could cause actual outcomes and results to differ materially. For a list and description of some of such risks and uncertainties, see our Annual Report on Form 10-K for the year ended December 31, 2017, including in the section thereof captioned Risk Factors , which is available at and The forward-looking statements in this presentation speak only as of the original date of this presentation and we undertake no obligation to update or revise any of these statements.
3 Note on Non-GAAP Financial Measures. This presentation includes non-GAAP financial measures that differ from financial measures calculated in accordance with generally accepted accounting principles (GAAP). These non-GAAP financial measures may not be comparable to similar measures reported by other companies and investors and other readers should consider these measures only as supplements to, and not as substitutes for or superior to, measures of financial performance prepared in accordance with GAAP. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures are included in the Appendix to this presentation.
4 Forward-Looking Non-GAAP Financial Measures. This press release also includes certain forward-looking non-GAAP financial measures for the year ending December 31, 2018. We calculate forward-looking non-GAAP financial measures based on internal forecasts that omit certain amounts that would be included in GAAP financial measures. For instance, we exclude the impact of certain potential charges or gains connected to quality enhancement and remediation efforts and certain legal and tax matters. Other than projected free cash flow for the year ending December 31, 2018, for which a reconciliation is provided, we have not provided quantitative reconciliations of these forward- looking non-GAAP financial measures to the most directly comparable forward-looking GAAP financial measures because the excluded items are not available on a prospective basis without unreasonable efforts.
5 It is probable that these forward-looking non-GAAP financial measures may be materially different from the corresponding GAAP financial measures. 2. Table of Contents 2018 Q1 Financial Summary 2018 Guidance & Outlook Company Overview Appendix 3. 2018 Q1 Financial Summary 1st Quarter 2018 Net Sales Results Product Category Performance Q1. Q1. Net Sales Constant Currency Product (in millions). (3). (1). % Change (1). (3). Knees $713 Hips 492 443 Dental 108 Spine & CMF 183 Other 79 Total $2,018 (1). (2). Geographic Region Performance Q1. Q1. Net Sales Constant Currency Region (in millions) % Change (1). (3) (3). Americas $1,208 Europe 497 Asia Pacific 313 Total $2,018 (1) Non-GAAP financial measure; see reconciliation.
6 5. Knees & Hips Product Categories Persona Q1. Personalized Knee system Revenue Constant Currency (in m illions) (1). % Change KNEES Americas EMEA. $ 417. 189. APAC 107 Total Knees $ 713 Persona . Partial Knee system Strong growth from Persona The Personalized Knee system Continued positive reception of the Persona Partial Knee launched in fall of 2017. Persona TM Tibia FDA clearance and CE Mark received as well as first surgical case performed early 2018. Q1. taperloc complete Hip system Revenue Constant Currency (in m illions) (1). % Change HIPS Americas EMEA. $ 248. 142. APAC 102 Total Hips $ 492 G7 Acetabular system Positive growth of taperloc complete Hip system and G7 Acetabular system Sales of Arcos Modular Femoral Revision system continued growth Warsaw North Campus supply constraints linger, but steadily improving (1) Non-GAAP financial measure; see reconciliation 6.
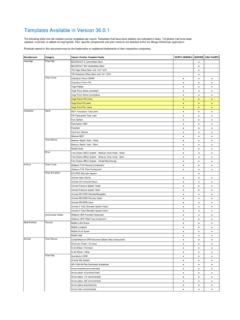
7 Product Category Q1. Revenue Constant Currency GLOBAL REVENUE (in m illions) % Change (1). $443 IntelliCart system Steady demand for wound debridement and skin graft portfolios SURGICAL Strong growth for Universal Power system surgical instruments Gel-One . Specialized sales representatives focused on driving growth SPORTS Cross-Linked Hyaluronate Warsaw North Campus supply constraints linger MEDICINE Steady growth from BioCUE brand Sidus . Stem-Free Shoulder Continued success of Comprehensive Total Shoulder system EXTREMITIES Full launch of Sidus Stem-Free Shoulder as well as a limited launch of Comprehensive Augmented Baseplate in Q1 2018.
8 Total Foot system Continued buildout of specialized sales force FOOT & ANKLE Steady demand for system portfolio Continued market acceptance for Subchondroplasty Procedures NCB Periprosthetic Strong sales of the DVR Distal Radius Plating system Portfolio Femur Plating system Continued success of Zimmer Natural Nail system Portfolio TRAUMA Steady growth of NCB brands led by the NCB Periprosthetic Plating Systems (1) Non-GAAP financial measure; see reconciliation 7. Spine, CMF & Thoracic and Dental Product Categories Q1. SPINE & CMF Revenue Constant Currency (1). (in m illions) % Change GLOBAL REVENUE Spine & CMF $183 Mobi-C Cervical Disc Growth of Vitality Spinal Fixation platform contributed to Q1 sales SPINE Continued strong market acceptance of Mobi-C Cervical Disc Remain focused on resolution of salesforce integration dis-synergies SternaLock Continued strong demand for the SternaLock Blu Primary Closure CMF & Blu Closure system and RibFix Blu Thoracic Fixation system THORACIC system FDA 510(k) clearance of ROSA Brain Q1.
9 DENTAL Revenue Constant Currency (in m illions) % Change (1). GLOBAL REVENUE Dental $108 Osseotite Realigning portfolio to meet market needs DENTAL Implant Continued restructuring of sales organization in certain key markets (1) Non-GAAP financial measure; see reconciliation 8. Innovative Solutions Across the Continuum of Care Recently Launched Sidus Stem-Free Shoulder (Introduced February 2018 - US). Total shoulder arthroplasty solution Available in EMEA since 2012, now available in US. Innovating to: with recent FDA clearance and launch Sidus . Second stemless shoulder on the market Stem-Free Shoulder Minimize Complications Product Pipeline Reduce Surgery Times Persona TM Tibia Knee (2H 2018).
10 Improve Efficiencies Cementless knee utilizing clinically proven Trabecular MetalTM Technology FDA clearance/CE Mark received and first surgical case performed early 2018. Optimize Outcomes Persona Revision Knee (2H 2018 Limited Launch). Enhance Patient Adds revision offering to Persona portfolio ROSA . Experiences ROSA Knee (2H 2018 Limited Launch) Knee Timeline remains on track Global commercialization strategy complete Key Design Principles: X-Ray based, time net neutral, soft tissue balancing Comprehensive Augmented Baseplate (Mid 2018). FDA clearance received in early 2018 First surgery March 2018. Complements Comprehensive Reverse Shoulder system by offering alternative to bone graphing and eccentric reaming for patients with glenoid defects Currently in limited launch with full launch expected in June 2018.