Consider The Reaction 2
Found 10 free book(s)Continuous Stirred Tank Reactors (CSTRs)
ocw.mit.eduAoA A CX r τ= − Consider: 1st Order Reaction Kinetics −=rkCAA concentration or conversion? Æ convert rate law from C A to X A CC XAAo A=−()1 −= −rkC XAAo()1 A ()11() Ao A A reactor size in terms of conversion and rate constant Ao A A CX X kC X k X τ ⎫⎪ ==⎬ −−⎪⎭ Æ rearrange to find how much conversion for a given reactor size
The Basics of Reaction Kinetics for Chemical Reaction ...
authors.library.caltech.edu4 CHAPTER 1 The Basics of Reaction Kinetics for Chemical Reaction Engineering The next task in describing a chemically reacting system is the identifica tion of the reactions and their arrangement in a network. The kinetic analysis of
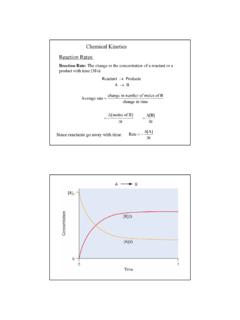
Chemical Kinetics Reaction Rates - csus.edu
www.csus.edu5 The Overall Order of a reaction is the sum of the individual orders: Rate (Ms−1) = k[A][B]1/2[C]2 Overall order: 1 + ½ + 2 = 3.5 = 7/2 or seven−halves order note: when the order of a reaction is 1 (first order) no exponent is written. Units for the rate constant: The units of a rate constant will change depending upon the overall
Chemical Reactions Name - sciencespot.net
www.sciencespot.netT. Trimpe 2010 http://sciencespot.net/ Chemical Reactions Name _____ 1. Watch the video and then complete the chart. Type of Reaction Definition Equation Synthesis
Oxidation Reactions of Sugars - University Of Illinois
butane.chem.uiuc.eduHalf Reactions for Chlorate + Glucose Chlorate anion is another oxidizing agent (see the gummy worm reaction on the index page) that can oxidize glucose and become reduced. The oxidation of glucose to carbon dioxide is the same as above.
5.02 Kinetics of the Persulfate-iodide Clock Reaction
cartwright.chem.ox.ac.uk2nd/3rd Year Physical Chemistry Practical Course, Oxford University 5.02 Kinetics of the Persulfate-iodide Clock Reaction (4 points) In this experiment you will investigate the kinetics of the reaction between persulfate
EXPERIMENT 10: Precipitation Reactions
swc2.hccs.edu2 They are called spectator ions, or simply spectators. Canceling the spectator ions from both sides of an ionic equation remains the net ionic equation that includes only the substances and ions that actually remains in the reaction as water, gas, insoluble solid (precipitate), weak electrolyte, and no electrolyte.
Chapter 14: Acids and Bases - Ohio Northern University
www2.onu.eduThe reaction of interest is: OH-(aq) + H 2 O(l) H 2 O(l) + OH-(aq).Since there is no net change in the molecules present after the reaction, it is sufficient to know that the base dissociates in water.
Reaction Kinetics - vallance.chem.ox.ac.uk
vallance.chem.ox.ac.uk2 1. Introduction Chemical reaction kinetics deals with the rates of chemical processes. Any chemical process may be broken down into a sequence of one or more single-step processes known either as elementary processes, elementary reactions, or elementary steps.Elementary reactions …
Organic Chemistry 32-235 Practice Questions for Exam #2 …
www.uwosh.eduOrganic Chemistry 32-235 Practice Questions for Exam #2 Part 1: (Circle only ONE choice, circling more than one will be counted as wrong!) 4 points each 1. …
Similar queries
Continuous Stirred Tank Reactors CSTRs, Consider, Reaction, Reaction Kinetics for Chemical Reaction, Reaction Kinetics for Chemical Reaction Engineering, Oxidation Reactions of Sugars, Kinetics of the Persulfate-iodide Clock Reaction, The reaction, Precipitation, Chapter 14: Acids and Bases, Organic Chemistry 32-235 Practice Questions