Iso 10993 7
Found 5 free book(s)INTERNATIONAL ISO STANDARD 10993-1
nhiso.comISO 10993-6, Biological evaluation of medical devices — Part 6: Tests for local effects after implantation ISO 10993-7, Biological evaluation of medical devices — Part 7: Ethylene oxide sterilization residuals ISO 10993-9, Biological evaluation of medical devices — Part 9: Framework for identification and
Part 5: Tests for in vitro cytotoxicity
nhiso.comISO 10993-5 was prepared by Technical Committee ISO/TC 194, Biological evaluation of medical devices. This third edition cancels and replaces the second edition (ISO 10993-5:1999) which has been technically revised. ISO 10993 consists of the following parts, under the general title Biological evaluation of medical devices:
ISO 10993シリーズ収録規格一覧
webdesk.jsa.or.jpiso 10993-7:2008 医療機器の生物学的評価-第7部:酸化エチレン滅菌処理残留物 40,800 44,880 英・日対訳 ISO 10993- 7:2008/Cor 1:2009
BIOCOMPATIBILITY OF PLASTICS
www.zeusinc.comISO 10993 is intended to assist manufacturers and engineers in designing an appropriate testing program for their device. Testing details are thus specific to each device and its application though there may be testing commonalities for multiple device types.
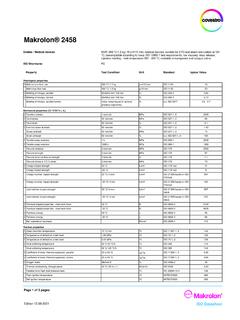
Makrolon® 2458 - Covestro
solutions.covestro.comMakrolon® 2458 Page 3 of 3 pages Edition 13.09.2021 ISO Datasheet Disclaimer Typical value These values are typical values only. Unless explicitly agreed in written form, the do not constitute a binding material specification or warranted values.