Reduced 4 Dose Vaccine Schedule
Found 8 free book(s)COVID-19 – mRNA Pfizer – Ultra Frozen Vaccine Adult 12 ...
www.albertahealthservices.careduced response to vaccines, will vary depending on the immunocompromising condition. Thus, a shortened interval no less than 28 days may be considered for ... them, and as a result received a mixed schedule, it is reasonable to not disadvantage ... vaccine series. • Dose 4 if needed – at least 28 days after the third dose for those with a ...
SOPs for reduced interval for 2nd dose for Covishield ...
www.mohfw.gov.in1. Presently, based on the recommendations by National Expert Group on Vaccine Administration for COVID-19 (NÉGVAC), the schedule of Covishield vaccine under National Covid-19 Vaccination Strategy is to administer the 2nd dose at 12-16 weeks interval (i.e. after 84 days), after administration of 1st dose. 2.
BOOSTRIX (Tetanus Toxoid, Reduced Diphtheria Toxoid and ...
gskpro.comlast dose of the Diphtheria and Tetanus Toxoids and Acellular Pertussis (DTaP) series or 5 years or more after a dose of Tetanus and Diphtheria Toxoids Adsorbed (Td). BOOSTRIX may be administered as an additional dose 9 years or more after the initial dose of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap).
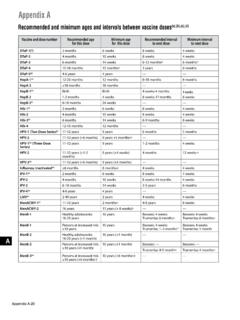
Appendix A: Schedule and Recommendations: …
www.cdc.govperiod may be used. If the third dose was administered on or after December 16, 2016, and was administered 12 weeks after the 2nd dose and 5 months after the first dose, it is a valid dose. The 4-day grace period may be used. (o) One dose of influenza vaccine per season is recommended for most persons.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
www.ema.europa.euOne single dose of 0.5 ml. Prevenar 13 vaccine schedule for infants and children previously vaccinated with Prevenar (7-valent) (Streptococcus pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) Prevenar 13 contains the same 7 serotypes included in Prevenar, using the same carrier protein CRM197.
Meningococcal vaccines for Australians - NCIRS
ncirs.org.audemonstrated the immunogenicity of MenACWY vaccine in children, adolescents and adults. All studies indicate that MenACWY vaccines are safe and immunogenic. 22-24 It is preferable to use the same brand of MenACWY vaccine to complete a primary vaccination course. Any brand of vaccine may be used as a booster dose.
Myocarditis and COVID-19 Vaccine Intervals
www.cdc.govFeb 04, 2022 · Within a 7-day risk period after either dose, the rate ratio of myocarditis for Moderna vaccine vs. an unvaccinated comparator was higher than Pfizer vaccine vs. an unvaccinated comparator – The highest rate ratios observed were among those receiving a second dose of Moderna in a heterologous mRNA primary series . VaST:
California Code of Regulations Title 17, Division 1, Chapter 4
eziz.orgEffective July 1, 2019 Page 1 of 13 IMM-1080 (9-18) California Code of Regulations Title 17, Division 1, Chapter 4 Subchapter 8. Immunization Against Poliomyelitis, Diphtheria, Pertussis, Tetanus,