Search results with tag "Rituximab"
DRUG NAME: Rituximab - BC Cancer
www.bccancer.bc.caRITUXAN® , SC RIXIMYO® (biosimilar), RUXIENCE® (biosimilar), TRUXIMO® (biosimilar) CLASSIFICATION: monoclonal antibody. Special pediatric considerations are noted when applicable, otherwise adult provisions apply. MECHANISM OF ACTION: Rituximab is a chimeric murine/human monoclonal antibody based on human immunoglobulin G (IgG). Rituximab
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
www.ema.europa.euIMBRUVICA as a single agent or in combination with rituximab or obinutuzumab is indicated for the treatment of adult patients with previously untreated chronic lymphocytic leukaemia (CLL) (see section5.1). IMBRUVICA as a single agent or in combination with bendamustine and rituximab (BR)is indicated
Bendamustine +Rituximab (BR) for CLL
nssg.oxford-haematology.org.ukBendamustine +Rituximab (BR) for CLL INDICATIONS 1. Treatment naïve TP53 wild-type CLL (NICE TA216) 2. Patients with relapsed B-cell chronic lymphocytic leukaemia (B-CLL) who are not eligible for treatment with BTK or BCL2 inhibitors. (not routinely commissioned by NHSE) NB.
Bendamustine +/- Rituximab (BR) for first line Chronic ...
www.londoncanceralliance.nhs.ukBendamustine +/- Rituximab (BR) for first line Chronic Lymphocytic Leukaemia Page 1 of 3 Reason for Update: New protocol Approved by Consultant: S Devereux Indication: First line treatment for B-CLL (Binet stage B or C) in patients for whom fludarabine combination chemotherapy is …
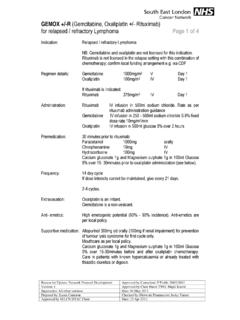
GEMOX +/-R (Gemcitabine, Oxaliplatin +/- Rituximab) for ...
www.londoncanceralliance.nhs.ukGEMOX +/-R (Gemcitabine, Oxaliplatin +/- Rituximab) for relapsed / refractory Lymphoma Page 1 of 4 Reason for Update: Network Protocol Development Approved by Consultant: P Fields 29/03/2012 Version: 1 Approved by Chair Haem TWG: Majid Kazmi
2.07 Protocol Name: CHOP & Rituximab - London Cancer …
www.londoncanceralliance.nhs.uk2 07 R-CHOP Version 2 0 Jul08.doc Page 1 of 5 2.07 Protocol Name: CHOP & Rituximab Indication • Intermediate and high grade, B-cell non-Hodgkins
Rituxan® (rituximab) Medication Precertification Request
www.aetna.comRituxan® (rituximab) Medication Precertification Request Page 1 of 3 . Aetna Precertification Notification . Phone: 1-866-752-7021 . FAX: 1-888-267-3277 . For Medicare Advantage Part B: (All fields must be completed and return both pages for precertification review) Please Use Medicare Request Form . Please indicate: Start of treatment, start ...
Repeated Administrations of Rituximab along with …
www.indianpediatrics.nettherapeutic option for refractory steroid-resistant nephrotic syndrome (SRNS). Bagga, et al. [1] reported the efficacy of treatment with four doses of RTX in five patients with nephrotic syndrome resistant to treatment with high-dose corticosteroids, alkylating agents, and calcineurin inhibitors [1]. In another study, a
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
www.ema.europa.euRituximab is a genetically engineered chimeric mouse/human monoclonal antibody representing a glycosylated immunoglobulin with human IgG1 constant regions and murine light -chain and heavy-chain variable region sequences. The antibody is produced by …
Venetoclax and Obinutuzumab
nssg.oxford-haematology.org.ukFirst line treatment of adult patients with previously untreated chronic lymphocytic leukaemia (CLL) or Small Lymphocytic Lymphoma (SLL): 1. In the presence of 17p deletion or TP53 mutation (NICE TA663- BLUETEQ required). OR 2. In the absence of 17p deletion or TP53 mutation in adult patients for whom Fludarabine, Cyclophosphamide and Rituximab ...
Welcome to HUMIRA Complete. - humirapro.com
www.humirapro.comyou have ever used RITUXAN® (rituximab), IMURAN® (azathioprine), or PURINETHOL® (mercaptopurine, 6-MP). What should I watch for AFTER starting HUMIRA?, including: • Serious infections. These include TB and infections caused by viruses, fungi, or bacteria. Symptoms related to TB include a
Medications that are immunosuppressive or immunomodulatory
hartfordhealthcare.orgAug 16, 2021 · Rituximab Rituxan Monoclonal antibody Secukinumab Cosentyx Other biological agent Sirolimus Rapamune mTOR inhibitor streptozocin Zanosar Nitrosourea Tacrolimus Protopic, Envarsus XR Calcineurin Inhibitor temozolomide Temodar Alkylating agent Thioguanine Tabloid Antimetabolite . thiotepa Tepadina Alkylating agent ...
RITUXAN (RITUXIMAB) - UHCprovider.com Home
www.uhcprovider.comRituxan® treatment.
BCCA Protocol Summary for Treatment of Non-Hodgkin ...
www.bccancer.bc.caBCCA Protocol Summary for Treatment of Non-Hodgkin Lymphoma with Bendamustine and riTUXimab . Protocol Code . ULYBENDR
CHIMIOTHERAPIE des LYMPHOMES NON …
www.omedit-hautenormandie.fr3 R-CHOP Coiffier B et al N. Eng J. Med. 2002 Jan 24; 346(4): 235-42 J 1 Rang Voie METHYLPREDNISOLONE 40 mg/m2 1 IV RITUXIMAB 375 mg/m2
Reference ID: 3366104 - Food and Drug …
www.accessdata.fda.gov2.1. Overview of Dosing Schedule 2.2. Zevalin Therapeutic Regimen Dosage and Administration . Day 1: • Premedicate with acetaminophen 650 mg orally and diphenhydramine 50 mg orally prior to rituximab infusion.
Similar queries
Rituximab, RITUXAN, Chronic lymphocytic leukaemia, Bendamustine, Bendamustine +/- Rituximab (BR) for first, Bendamustine +/- Rituximab (BR) for first line Chronic Lymphocytic Leukaemia, GEMOX +/-R Gemcitabine, Oxaliplatin +/- Rituximab, GEMOX +/-R (Gemcitabine, Oxaliplatin +/- Rituximab) for relapsed / refractory, Protocol Name: CHOP & Rituximab, CHOP, RITUXAN® rituximab, Aetna, Repeated Administrations of Rituximab along, Steroid, Resistant nephrotic, Treatment, Nephrotic, Resistant, SUMMARY OF PRODUCT CHARACTERISTICS, First line, Lymphocytic, Rituximab Rituxan, RITUXAN RITUXIMAB, Non-Hodgkin Lymphoma with Bendamustine and riTUXimab, Food and Drug