Search results with tag "Prevnar"
MATERIAL SAFETY DATA SHEET - Pfizer
www.pfizer.com1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Material Name: Prevnar 13 Trade Name: Prevnar 13 Synonyms: Pneumococcal 13-Valent Conjugate Vaccine
Bexsero Product Monograph - GSK
ca.gsk.comvalent Conjugate Vaccine, Diphtheria CRM197 Protein (PREVNAR) and diphtheria, tetanus, acellular pertussis, hepatitis B recombinant (adsorbed), inactivated poliomyelitis and adsorbed conjugated . Haemophilus influenzae . type b vaccine (INFANRIX HEXA)]. Fever occurred more
What is Pneumonia? - American Thoracic Society
www.thoracic.orgbacteria (Prevnar 13® and/or Pneumovax 23® pneumococcal vaccines) are also at higher risk for lung infections. What are the signs and symptoms of pneumonia? People with pneumonia often have a cough, fever or chills, difficulty breathing, low energy and poor appetite. Sometimes a person will have nausea, diarrhea, and/or chest pain. It is
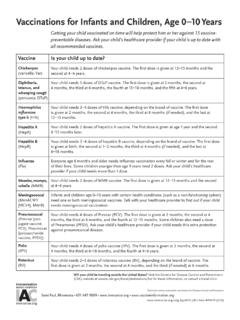
Vaccinations for Infants and Children, Age 0-10 Years
www.immunize.orgYour child needs 4 doses of Prevnar (PCV13). The first dose is given at 2 months, the second at Pneumococcal 4 months, the third at 6 months, and the fourth at 12–15 months. Some children also need a dose of Pneumovax (PPSV23). Ask your child’s healthcare provider if your child needs this extra protection against pneumococcal disease.
07.291 Pneumococcal 13-valent Conjugate Vaccine Biological ...
albertahealthservices.cainflammatory arthropathies, e.g., systemic lupus erythematosus (SLE), rheumatoid or juvenile arthritis, Alberta Health Services Standard #07.291 September 5, 2018 Immunization Program Standards Manual Page . 2. of . 9. Population, Public and Indigenous Health . Prevnar® 13
Vaccine Excipient Summary-Excipients Included in U.S ...
www.cdc.gov(PCV13 – Prevnar 13) 8/2017 . CRM. 197. carrier protein, polysorbate 80, succinate buffer, aluminum phosphate . Pneumococcal (PPSV-23 – Pneumovax) 2/2020* isotonic saline solution, phenol : Polio (IPV – Ipol) 2/2020* calf bovine serum albumin, 2-phenoxyethanol, formaldehyde, neomycin,
PCV13 vs PPSV23: Which to Give and When
www.medicine.wisc.eduObjectives • Understand the indications for adults for PPSV23 (Pneumovax) • Understand the indications for adults for PCV13 (Prevnar)
PREVNAR 13 REIMBURSEMENT RESOURCE ShEET
www.vrablehealthcare.com• Prevnar 13® is a vaccine indicated for active immunization for the prevention of disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F