Search results with tag "Monographs"
IARC Monographs on the Evaluation of Carcinogenic Risks to ...
monographs.iarc.who.intSection of IARC Monographs, International Agency for Research on Cancer, 150 cours Albert Thomas, 69372 Lyon Cedex 08, France, in order that the agent may be considered for re-evaluation by a future Working Group. Although every effort is made to prepare the monographs as accurately as possible, mistakes may occur.
General Notices, Monographs, General Chapters, Reagents ...
www.uspnf.comGeneral Notices, Monographs, General Chapters, Reagents, and Tables Affected by Changes Appearing in NF 35 Page citations refer to the pages of NF 35. Note—In the list below, if a section is new or if a subsection is added to or deleted from an existing section, it is labeled as such in parentheses after the section or subsection name.
IARC MONOGRAPHS ON THE EVALUATION OF …
monographs.iarc.who.intto include consideration of exposures to complex mixtures of chemicals (which occur, for example, in some occupations and as a result of human habits) and of exposures to other agents, such as radiation and viruses. With Supplement 6 (IARC, 1987a), the title of the series was modified from IARC Monographs on the Evaluation of the Carcino-
USP 39 Official Monographs / Calcium 1
www.uspnf.comUSP 39 Official Monographs / Calcium 1. Acceptance criteria: NMT 10 ppm Calcium Citrate • LIMIT OF FLUORIDE [NOTE—Prepare and store all solutions in plastic containers.] Standard stock solution: 1000µg/mL of fluoride ion from USP Sodium Fluoride RS in water Standard solution: 5µg/mL of fluoride ion from Stan-dard stock solution.
IARC Monographs evaluate consumption of red meat and ...
www.iarc.who.intPRESS RELEASE N° 240 26 October 2015. IARC Monographs evaluate consumption of red meat and processed meat . Lyon, France, 26 October 2015 – The International Agency for Research on Cancer (IARC), the cancer agency of the World Health Organization, has the carcinogenicity of evaluated the consumption of red
ALOE VERA - IARC Monographs on the Identification of ...
monographs.iarc.who.intIARC MONOGRAPHS – 108 40 been traditionally used as the laxative (Eur Ph, 2008). (a) Aloe vera gel extract The inner leaf pulp of the Aloe vera plant contains large, thin-walled cells that produce gel, the clear, mucilaginous, and aqueous extract of the inner central area of the leaf pulp (Fig. 1.2). Aloe vera gel serves as the water and energy
OFFICIAL, EFFECTIVE DATE 11/04/2021
www.usp.orgscope at this time are pending monographs for excipients, and novel excipients. At such time as official procedures are developed for those items, they will be included in this guideline. Please contact USP Customer Engagement for Excipients. for additional information concerning questions for items that are out of scope. 3.
Official Monographs - Pmda
www.pmda.go.jpthe mobile phase, add exactly 10 mL of the internal standard solution and the mobile phase to make 50 mL, and use this solution as the sample solution. Separately, weigh accurately about 45 mg of Aceglutamide RS, dissolve in a suitable amount of the mobile phase, add exactly 10 mL of the inter-nal standard solution and the mobile phase to make ...
EXPOSURE TO ARSENIC: A MAJOR PUBLIC HEALTH CONCERN
www.who.intLyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100) [summary in Straif K et al. (2009). A review of human carcinogens—Part C: Metals, arsenic, dusts, and fibres.
Hazard Communication Standard: Safety Data Sheets
www.osha.govInternational Agency for Research on Cancer (IARC) Monographs (latest editions) or found . to be a potential carcinogen by OSHA. 5. Section 12: Ecological Information (non-mandatory) This section provides information to evaluate the environmental impact of the chemical(s) if it .
Impactos ambientales del petróleo - Greenpeace
www.greenpeace.org5.- IARC. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: occupational exposures to petroleum refining; crude oil and major petroleum fuels. Volume 45. IARC, Lyon 1989. 6.- Campbell D, Cox D, Crum J, Foster K, Christie P. Initial effects of the grounding of the tanker Braer on health in Shetland.
MATERIAL SAFETY DATA SHEET
media.distributordatasolutions.comMay 02, 2018 · IARC Monographs on the Evaluation of Carcinogenic Risks to Humans No carcinogenic components identified US. National Toxicology Program (NTP) Report on Carcinogens: No carcinogenic components identified Product Name: RIDGID Dark Thread Cutting Oil (United States)
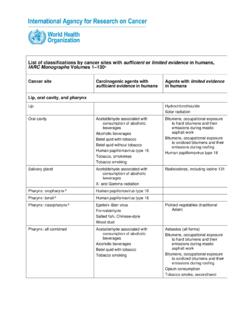
List of classifications by cancer sites with sufficient or ...
monographs.iarc.who.intIARC Monographs Volumes 1–130a. Cancer site . Carcinogenic agents with . sufficient evidence. in humans . Agents with . limited evidence. in humans . Lip, oral cavity, and pharynx; Lip . Hydrochlorothiazide . Solar radiation : Oral cavity . Acetaldehyde associated with …
SAFETY DATA SHEET - Sprayway, Inc
www.spraywayinc.comIARC Monographs on the Evaluation of Carcinogenic Risks to Humans: No carcinogenic components identified US. National Toxicology Program (NTP) Report on Carcinogens: No carcinogenic components identified US. OSHA Specifically Regulated Substances (29 CFR 1910.1001-1050): No carcinogenic components identified Germ Cell Mutagenicity In vitro
シンレキ工業株式会社|エムコール|ロードプラスターK| …
www.shinreki.co.jpIARC ( 1985) Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Vol.35 SUPPLEMENT 7 No.176 (2009.7) 044—366— 1617 044—366—1618 kenkyu@shinreki.co.jp El 7/7 8 17: 30 . Created Date:
POLYVINYL ALCOHOL (PVA)
www.fao.orgIARC Working group, feb. 7-13,1978,Lyon, France. World Health Organization (WHO). International Agerny for Research on Cancer (IARC); Lyon, France. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man, Vol. 19, pp. 341-366. Title: Microsoft Word - 2004-02-24 CTA 61 PVA.doc Author: ML_Admin
PRODUCT MONOGRAPH INCLUDING PATIENT …
ca.gsk.comPRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION SHINGRIX Herpes Zoster vaccine (non-live recombinant, AS01B adjuvanted) Suspension for injection, 50 mcg Varicella Zoster Virus (VZV) glycoprotein E (gE), Intramuscular Injection Active Immunizing Agent GlaxoSmithKline Inc. 7333Mississauga Road Mississauga, Ontario L5N 6L4
PRODUCT MONOGRAPH INCLUDING PATIENT …
covid-vaccine.canada.caPRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrCasirivimab and imdevimab for injection Solutions for infusion, each available as 1332 mg/11.1 mL and 300 mg/2.5 mL (120mg/mL) single-use vials Anti-SARS-CoV-2 spike protein monoclonal antibodies HEALTH CANADA HAS AUTHORIZED THE SALE OF THIS COVID-19 DRUG BASED ON …
PRODUCT MONOGRAPH INCLUDING PATIENT …
ca.gsk.comPRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION ENGERIX-B Hepatitis B vaccine (recombinant) 0.5 mL and 1.0 mL suspensions of 20 mcg/mL hepatitis B surface antigen for injection Active immunizing agent against infection caused by allknown subtypes of hepatitis B virus ATC Code: J07BC01 GlaxoSmithKline Inc. 7333 Mississauga …
Fluzone High Dose - Sanofi
products.sanofi.caSanofi Pasteur Product Monograph 372 - FLUZONE® High-Dose Page 3 of 23 FLUZONE® High-Dose Influenza Virus Vaccine Trivalent Types A and B (Split Virion) PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration: Intramuscular injection. Dosage Form/Strength: Suspension for injection. Active …
PRODUCT MONOGRAPH - Pfizer Canada
www.pfizer.caPrevnar® 13 (Pneumococcal 13-valent Conjugate Vaccine) Product Monograph Page 7of 61 3. Adults 18Years and Older Safety was assessed in 7 clinical studies including 91,593 adults ranging in ages from 18 to 101 years. Prevnar 13 was administered to 48,806 adults; 2,616 adults were aged 50-64 years and 45,291 adults were 65 years and older.
PART III: CONSUMER INFORMATION INSPIOLTO RESPIMAT …
www.boehringer-ingelheim.caInspiolto® Respimat ® Product Monograph Page 43 of 50 PART III: CONSUMER INFORMATION ... CONSUMER INFORMATION . ... including drugs prescribed by other doctors, vitamins, minerals, natural supplements, or alternative medicines. ...
PRODUCT MONOGRAPH SYMBICORT …
www.astrazeneca.caindicated for the maintenance treatment of moderate to severe COPD including chronic bronchitis and emphysema, in patients with persistent symptoms and a history of exacerbations, where the use of a combination product is considered appropriate. SYMBICORTTURBUHALER is NOT indicated for the relief of acute bronchospasm in COPD patients.
PATIENT MEDICATION INFORMATION - AstraZeneca
www.astrazeneca.caProduct Monograph Master Template Template date: September 2020 FORXIGA (dapagliflozin propanediol monohydrate) Page 1 of 8 COPYRIGHT 2014-2021 ASTRAZENECA CANADA INC PATIENT MEDICATION INFORMATION READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PrFORXIGA Dapagliflozin tablets
PRODUCT MONOGRAPH INCLUDING PATIENT …
pdf.hres.caPRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION AFLURIA® TETRA Quadrivalent Inactivated Influenza Vaccine (Split Virion) ... Product Monograph Page 8 of 24 In children 5 to < 18 years of age, the most commonly reported injection-site adverse reactions observed in clinical studies with AFLURIA® TETRA were pain (51.4%), redness (17.1 ...
PRODUCT MONOGRAPH
pdf.hres.caPRODUCT MONOGRAPH DUKORAL® Oral, Inactivated Cholera and ETEC Diarrhea Vaccine Oral Suspension Active Immunizing Agent for the Prevention of Diarrhea Caused by Vibrio cholerae and/or heat-labile toxin producing Enterotoxigenic Escherichia coli ATC Code: J07AE01 Manufactured by: Date of Approval: Valneva Sweden AB,
Standardized Adult (40-130 kg) IVIG Infusion Rate Tables ...
www.gov.nl.caFor complete product information please refer to product monograph. Rate mL/kg/hr Patient Weight in Kilograms 40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115 120 125 130 Infusion Rate mL/hr 0.3 12 13.5 15 16.5 18 19.5 21 22.5 24 25.5 27 28.5 30 31.5 33 34.5 36 37.5 39
10 pct Adult IVIG Infusion Rate Table
www.albertahealthservices.caFor complete product information, refer to the product information monograph. ** High infusion rates should not be used for patients at risk for renal impairment, elderly patients, or ... 75 38 113 225 375 450 600 80 40 120 240 400 480 640 85 43 128 255 425 510 680 90 45 135 270 450 540 720 95 48 143 285 475 570 760 ...
FLUZONE Quadrivalent
pdf.hres.caSanofi Pasteur Product Monograph 450/477 - FLUZONE® Quadrivalent Page 4 of 35 FLUZONE® Quadrivalent Influenza Virus Vaccine Quadrivalent Types A and B (Split Virion) PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration: Intramuscular injection. Dosage Form/Strength: Suspension for injection.
PRODUCT MONOGRAPH
pdf.hres.caPRODUCT MONOGRAPH Pr WELLBUTRIN XL Bupropion Hydrochloride Extended-Release Tablets, USP 150 mg and 300 mg Antidepressant Name: Valeant Canada LP Address: 2150 St-Elzear Blvd. West Laval, QC, H7L 4A8 Date of Revision: March 20, 2017 Control Number: 200959
PRODUCT MONOGRAPH INCLUDING PATIENT …
covid-vaccine.canada.caPRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION SPIKEVAX™ Elasomeran mRNA vaccine Dispersion for intramuscular injection Multidose Vial, 100 mcg / 0.5mL Active Immunizing Agent Submission Control Number: 259289 ModernaTX, Inc. 200 Technology Square Cambridge, MA, USA, 02139 Imported and Distributed by: Innomar Strategies, Inc.
PRODUCT MONOGRAPH INCLUDING PATIENT …
pdf.hres.caRhesus alloimmunisation The use of Mifegymiso requires measures to prevent rhesus alloimmunisation. Serious Warnings and Precautions It is important that all patients be followed by a physician 7 to 14 days after taking mifepristone to confirm safety and complete pregnancy termination (see WARNINGS AND
PRODUCT MONOGRAPH INCLUDING PATIENT …
www.astrazeneca.caProduct Monograph Master Template Template date: September 2020 FORXIGA (dapagliflozin propanediol monohydrate) Page 1of 75 COPYRIGHT 2014-2021 ASTRAZENECA CANADA INC PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION FORXIGA® Dapagliflozin propanediol monohydrate Tablets, 5 mg and 10 mg, Oral ATC Code: A10BK01
PRODUCT MONOGRAPH INCLUDING PATIENT …
www.caddra.caPRODUCT MONOGRAPH . INCLUDING PATIENT MEDICATION INFORMATION . FOQUEST® methylphenidate hydrochloride . Controlled Release Capsules . 25 mg, 35 mg, 45 mg, 55 mg, 70 mg, 85 mg and 100 mg . Professed Standard . Central Nervous System Stimulant . Purdue Pharma 575 Granite Court
PRODUCT MONOGRAPH INCLUDING PATIENT …
www.novonordisk.caProduct Monograph OZEMPIC® semaglutide injection Page 1 of 56. PRODUCT MONOGRAPH . INCLUDING PATIENT MEDICATION INFORMATION . PROZEMPIC® semaglutide injection . 2 mg/pen (1.34 mg/mL) 4 mg/pen (1.34 mg/mL) Pre-filled pen delivering doses of 0.25 mg or 0.5 mg . and . Pre-filled pen delivering doses of 1 mg . ATC code: A10BJ06 ...
PRODUCT MONOGRAPH
pdf.hres.caResponse to all diabetic therapies should be monitored by periodic measurements of blood glucose and HbA1c levels, with a goal of decreasing these levels towards the normal range. HbA1c is especially useful for evaluating long-term glycemic control.1 Sitagliptin is substantially excreted by the kidney.
Monograph ID / INCI Names (12/18/2020)
eservices.personalcarecouncil.orgMonograph ID / INCI Names (12/18/2020) 214 Ascorbyl Dipalmitate 215 Ascorbyl Palmitate 216 Ascorbyl Stearate 217 Asparagine 218 Aspartic Acid 219 Attapulgite
How to use the BP - British Pharmacopoeia
www.pharmacopoeia.compreparation BP monograph can be found in the next section of this guide. Herbal Drugs, Herbal Drug Preparations and Herbal Medicinal Products / Materials for use in the Manufacture of Homoeopathic Preparations / Blood-related Products / Immunological Products / Radiopharmaceutical Preparations / Surgical Materials
This document is referenced in USP General Chapter <1121 ...
www.usp.orgthe monograph title (e.g. Acetaminophen and Codeine Phosphate Tablets). o Where the vehicle is therapeutically active or equivalent to another component, the word “and” is used in the monograph title (e.g., Dextrose and Sodium Chloride Injection). • Use of the term “in”:
Guideline on specifications: test procedures and ...
www.ema.europa.eu69 specifications: test procedures and acceptance criteria for herbal substances, herbal preparations and 70 herbal medicinal products/traditional herbal medicinal products’ were introduced, which take into 71 account new and revised guidelines, the European Pharmacopoeia revised general monograph ‘Herbals
products1/traditional herbal medicinal products
www.ema.europa.euthe European Pharmacopoeia revised general monograph ‘Herbals Drugs’, as well as new requirements for impurities. Given the nature of this update, a concept paper or public consultation ... for human herbal medicinal products and Part 2 of Annex I to Directive 2001/82/EC as amended for
Medicinal Uses for Herbal Teas: Evidence, Dosing, and ...
www.fammed.wisc.eduSep 28, 2007 · This monograph evaluates the scientific evidence for 10 common herbal tea preparations and their effectiveness. There are many other common herbal teas used medicinally; these 10 were chosen as the focus of this Supplement Sampler edition for their good safety profiles, lower drug-herb interaction
The Globalisation of the Pharmaceutical Industry Monograph
www.ifpma.orgPharmaceuticals Policy and Law 18 (2016) 1–4 1 DOI 10.3233/PPL-160427 IOS Press Editorial – The innovation and access to landscape Jose Luis Valverdea,∗ and Eduardo Pisanib aChair Jean Monnet of EU Law, Granada, Spain bInternational Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Geneva, Switzerland
Overview of USP General Chapters <476> and <1086>
www.usp.orgOct 19, 2017 · radiopharmaceuticals, herbal and crude products of animal or plant origin Introduces the following limits: ... and that monograph procedure does not detect an impurity present in the substance, the amount and identity of the impurity, where both are known, shall be stated in the labeling (certificate of analysis) of the official substance ...
REVISION OF MONOGRAPH ON TABLETS - World Health …
www.who.intmass of tablet cores before coating. In-process controls during tablet production should also include the dimensions (thickness, diameter), uniformity of mass, hardness and/or crushing force, friability, disintegration, or dissolution rate (for example, for modified-release tablets) of the finished dosage form.
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION …
covid-vaccine.canada.caThe Adenovirus type 26 (Ad26) vectored COVID-19 vaccine encoding the SARS-CoV-2 Spike (S) protein is produced in the PER.C6® TetR Cell Line and by recombinant DNA technology. Janssen COVID-19 Vaccine is supplied as a suspension in a multi-dose Type I glass vial with a
ENGERIX-B
pdf.hres.caPRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION ENGERIX-B Hepatitis B vaccine (recombinant) 0.5 mL and 1.0 mL suspensions of 20 mcg/mL hepatitis B surface antigen for injection Active immunizing agent against infection caused by all known subtypes of hepatitis B virus ATC Code: J07BC01 GlaxoSmithKline Inc. 7333 Mississauga Road
Question: What conversion ratio is recommended between ...
olh.ieThe product information for pregabalin and gabapentin suggest that both agents ... 2101-2700 450 2700 or higher 600 ... Monograph Pregabalin. British National Formulary. Available at www.medicinescomplete.com. Accessed on the 28/11/2016. Page 5 of 5
Similar queries
Monographs, IARC Monographs on the Evaluation of, Exposures, Official Monographs, IARC Monographs, International Agency for Research, IARC, Aloe vera, Official, Mobile, Safety Data Sheets, Greenpeace, Classifications by cancer sites with, SAFETY DATA SHEET, POLYVINYL ALCOHOL PVA, PRODUCT MONOGRAPH INCLUDING PATIENT, SHINGRIX, ENGERIX, FLUZONE, PRODUCT MONOGRAPH, INFORMATION, PRODUCT INFORMATION, Including, CONSUMER INFORMATION, Product, PATIENT MEDICATION INFORMATION, PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION, Monograph, Quadrivalent, Product Monograph 450, Alloimmunisation, PRODUCT MONOGRAPH . INCLUDING PATIENT MEDICATION INFORMATION, INCLUDING PATIENT, Diabetic, Monograph ID / INCI Names 12, Ascorbyl, How to use the BP, Herbal, Referenced in USP General Chapter, Medicinal Uses for Herbal Teas, REVISION OF MONOGRAPH ON TABLETS, World Health, Cores, Adenovirus type, Type, PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION ENGERIX, Conversion ratio is recommended between