Search results with tag "Human subjects"
Research Project Notification and Human Subjects ...
www.umuc.eduHuman Subjects Approval Form 1 Procedures for Completing the Research Project Notification and Human Subjects Protection Form As outlined in UMUC Policy 130.25, Conducting Research Involving Human Subjects, all UMUC
Protecting Human Research Participants
grants.nih.govSep 26, 2018 · Goals and Principles of Human Subjects Protection Human subjects are essential to the conduct of research intended to improve human health. As such, the relationship between investigators and human subjects is critical and should be based on honesty, trust, respect. Historical Events Nazi Medical War Crimes (1939-1945)
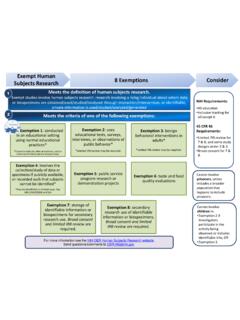
1 Meets the definition of human subjects research.
grants.nih.govJan 15, 2020 · NIH Exempt Human Subjects Research. 8 Exemptions. Meets the definition of human subjects research. Exempt studies involve human subjects research: research involving a living individual about whom data
Protecting Human Subjects Involved in Research
tracs.unc.eduJun 25, 2013 · Human Subject Protection Human Subject Protection: 1. Risks to Human Subjects 2. Adequacy of Protection Against Risks 3. Potential Benefits of Proposed Research 4. Importance of the Knowledge to be Gained 5. Data and Safety Monitoring Plan NIH grant applications must include a section titled Human Subjects Protection.
INFORMED CONSENT FOR HUMAN SUBJECTS RESEARCH
www.research.va.govThe Belmont Report of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research identifies three basic ethical principles that should serve as guideposts for human subjects research: respect for persons, beneficence, and jus tice. Truly informed consent upholds all three of these basic ethical principles.
A GUIDE TO RESEARCH ETHICS - University of Minnesota
www.ahc.umn.edu6 subjects. The Animal Welfare Act provides guidelines and regulations for research with animals. It goes into detail about sale, licensure, facilities, transport, and other care instructions. For research with human subjects Title 45, Part 46 from the Code of Federal Regulations (45 CFR 46): The Protection of Human Subjects Regulations
Guidelines for Reviewers: Protections for Human Subjects ...
grants.nih.govMar 18, 2019 · Guidelines for Reviewers: Protections for Human Subjects Review Criterion . Revision Notes — March 2019 • Revised definition for a Human Subject, in accordance with changes to the Common ... clinical trials . Background and References . …
Compensation of Research Subjects
cphs.berkeley.eduCommittee for Protection of Human Subjects University of California, Berkeley CPHS Guidelines – Compensation Page 2 of 6 July 2017 . obtain compliance. For example, a researcher might offer a month’s salary to subjects for one-day participation in a study to test the effects of an investigational drug with potentially serious side effects.
THE BELMONT REPORT: ETHICAL PRINCIPLES AND …
videocast.nih.govProtection of Human Subjects of Research." The identification of basic ethical principles that should underlie the conduct of research involving human subjects, and the development of guidelines to assure that such principles are followed, were topics of studies set forth in the Com- mission's mandate under Public Law 93-348. This mandate also
A Selected List of Unethical Human Subjects Research ...
www.gc.cuny.eduThe Stanford prison experiment was a psychological study of human responses to captivity and its behavioral effects on both authorities and inmates in prison. The experiment was conducted in 1971 by ... of the subjects was infringed during the study, "Tearoom Trade" has caused a major debate on privacy
2020 International Compilation of Human Research …
www.hhs.govgovern human subject protections in 133 countries, as well as standards from a number of international and regional organizations. First published in 2005, the Compilation is intended for use by researchers, IRBs/Research Ethics Committees, sponsors, and others who are involved in human subjects research protections around the world.
DOD INSTRUCTION 3216 - Washington Headquarters Services
www.esd.whs.milmonitor a component human research protection program (HRPP) management plan (CMP) in order to conduct or support DoD research involving human subjects, such as the Defense Health Agency, the National Security Agency, the Defense Intelligence Agency, the DoD Human
Guidance Document: Part C, Division 5 of the Food and Drug ...
www.canada.caTrials Involving Human Subjects” is the Food and Drugs Act (the Act). The Regulations came into force on September 1, 2001 and set out the federal requirements for the sale and importation of drugs used in human clinical trials in Canada, and include the requirement to comply with good clinical practices (GCP).
Ethical Considerations: The Common Rule
www.research.va.govthe Protection of Human Subjects” and was adopted by a number of federal agencies in 1991. Each agency incorporated the policy into its own code of Federal Regulations (CFR), with VA adapting it in Title 38 CFR Part 16, and the Department of Health and Human Services (DHHS) in 45 CFR Part 46, Subpart A.
INDIAN COUNCIL OF MEDICAL RESEARCH
ethics.ncdirindia.orgguidelines were revised in 2000 as the ‘Ethical Guidelines for Biomedical Research on Human Subjects’ under the chairmanship of Hon'ble Justice M N Venkatachaliah. In view of the new developments in the field of science and technology, another revision was carried out as Ethical Guidelines for Biomedical Research on Human Participants in 2006.
Ethics in Qualitative Research - Columbia University
www.columbia.eduappropriate ethical principles. Thus, the protection of human subjects or participants in any research study is imperative. Violations of human rights in the name of scientific research have been among the darkest events in history. From 1932-1972 more than 400 African American people who had
Institutional Review Board Guidebook - Saylor Academy
www.saylor.orgparticularly if the deception includes false feedback to the subjects about their own performance. Some examples from the American Psychological Association's guidebook, Ethical Principles in the Conduct of Research with Human Subjects (1973), illustrate the kinds of research - and the types of psychological risks - IRBs may encounter:
1 Meets the definition of human subjects research.
grants.nih.govor recorded such that subjects cannot be identified* *May be identifiable in limited cases. See §46.104(d)(4)(iii) and (iv) Exemption 5: public service program research or demonstration projects Exemption 6: taste and food quality evaluations Exemption 7: storage of identifiable information or biospecimens for secondary research use. Broad consent
Appendix on transparency rules to the Functional ...
www.ema.europa.euhuman subjects are publicly registered. The Regulation requires that in future all clinical trials used in support of a clinical trial application are publicly registered in a register providing data to the WHO ICTRP (WHO International Clinical Trials Registry Platform). Older clinical trials
International Ethical Guidelines for Health-related ...
cioms.chResearch Involving Human Subjects in 1993. The third version of the CIOMS Guidelines (2002) After 1993, ethical issues arose for which the 1993 CIOMS Guidelines had no specific provisions. They related mainly to externally sponsored clinical trials carried out in low-resource settings.
Similar queries
Research Project Notification and Human Subjects, Human Subjects, Research Project Notification and Human Subjects Protection, Research, Protecting Human Research Participants, Of Human Subjects Protection Human subjects, Human, Protecting Human Subjects Involved in Research, Protection of Human Subjects, RESEARCH ETHICS, Subjects, Guidelines, Clinical trials, Compensation, BELMONT REPORT, International Compilation of Human Research, Protection, Columbia University, Institutional Review Board, Exemption, Secondary, International Ethical Guidelines for Health