Search results with tag "Ideal gas"
U17- Particle Model: Ideal Gas Unit 17: Ideal Gas
faculty.une.eduU17- Particle Model: Ideal Gas Supported by NSF DUE 1044154 Jacques Charles into what we now call the Ideal Gas Law (PV=nRT)1.n=N/N A is the number of moles of "N" particles and "R" was the experimentally determined "ideal gas constant", 8.314 J/(moleK).
Calculation of Gas Density and Viscosity - CED Engineering
www.cedengineering.comis the ideal gas law constant, 8.3145 kg- m/kgmole-K . T. is the absolute temperature of the gas in K . With these units for . P, V, R, and T, the gas density will be in kg/m 3.. Critical Temperature and Pressure: As noted in the Introduction, in order to use the Ideal Gas Law to calculate a gas density, the gas temperature
The Ideal Gas Law Lecture 2: Atmospheric Thermodynamics
www.ess.uci.eduThe Ideal Gas Law An equation of state describes the relationship among pressure, temperature, and density of any material. All gases are found to follow approximately the same equation of state, which is referred to as the “ideal gas law (equation)”. Atmospheric gases, whether considered individually or as a
How to Measure Carbon Dioxide - Vaisala
www.vaisala.comdescribe the behavior of real gases. The ideal gas law relates the state of a certain amount of gas to its pressure, volume, and temperature, according to the equation: pV = nRT where p® = pressure [Pa] V = volume of the gas [m3] n = amount of gas [mol] R = universal gas constant (= 8.3145 J/mol K) T = temperature [K] Figure 2.
AP Chemistry Practice Questions Solids, Liquids and Gases
www.quia.comThe van der Waals equation of state is more descriptive for real gases. b. All gases behave the same way in the Ideal Gas Law. c. At a given T and V, one mole of Ne and CH 4 have the same pressure according to the Ideal Gas Law. d. The van der Waals equation corrects for deviations in the value of "R".
LECT05. Ideal Gas Calculations - Weebly
che31.weebly.com5 Ideal Gas Calculations Prof. Manolito E Bambase Jr. Department of Chemical Engineering. University of the Philippines Los Baños SLIDE 6 Example 5-1. Ideal Gas Calculation Solving for volumetric flowrate: n RT V= P Obtaining the molar flowrate from mass flowrate: 1100kg / h 19.0kmol/ h 58kg / …
Practice MC Test unit D (Ch 10) Gas Laws (pg 1 of 8)
www.livingston.org20. Real gases vary from the ideal gas laws gases at conditions of a. high temperature and low pressure b. both high temperature and high pressure c. both low temperature and low pressure d. low temperature and high pressure e. both high density and low pressure 21. If 2.0 moles of gas in a sealed glass flask is heated from 25ºC to 50ºC.
Polytropic Process of an Ideal Gas - Clarkson University
webspace.clarkson.eduLet an ideal gas undergo an infinitesimal adiabatic process: + =0 C V C dV p dp v results in: p Cp – Cv R Eliminating dT between these two equations and using PdV VdP nRdT results in PV nRT Taking the derivative of the ideal gas law: nC dT – PdV dU dQ – dW From the first law: dU nC dT, and dW PdV. dQ 0 v v = + = = = = = = =
Lecture 4: Pressure and Wind
www.ess.uci.eduThe Ideal Gas Law An equation of state describes the relationship among pressure, temperature, and density of any material. All gases are found to follow approximately the same equation of state, which is referred to as the “ideal gas law (equation)”. Atmospheric gases, whether considered individually or as a
Conservation Laws - MIT
web.mit.eduintroduced, the ideal gas law can be written as, p =(γ−1) ρE − 1 2 ρ(u2 +v2) , (81) where γis the ratio of specific heats (for air, γ≈ 1.4). You may be more familiar with the ideal gas law in the form, p =ρRT where R is the gas constant and T is the temperature. Equation 81 is equivalent to p =ρRT but Equation 81
PHYS 1401 General Physics I EXPERIMENT 11 BOYLE’s LAW …
www.austincc.eduThe ideal gas law PV = nRT (2) states that this constant (nRT)is proportional to the amount of ideal gas in the sam-ple (the number of moles, n) and the absolute temperature, T. The constant R in this equation is the universal gas constant which has a value of R = 8.31J/(mole.K) in SI units. Note that if T is held constant throughout the ...
Example Exercise 11.1 Gas Pressure Conversion
www.austincc.eduIdeal Gas Behavior. Since the temperature of each gas is 25 ° C, we know that the kinetic energy is the same for NH. 3. and NO. 2. At the same temperature, we know that lighter molecules move faster than heavier molecules. The molecular mass of NH. 3. is 17 amu and NO. 2. is 46 amu. Since NH. 3. is lighter than NO. 2, the ammonia
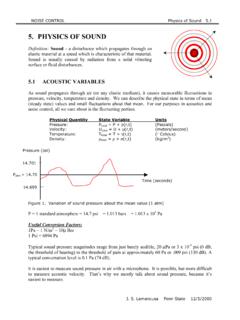
5. PHYSICS OF SOUND - Pennsylvania State University
www.mne.psu.eduJ. S. Lamancusa Penn State 12/5/2000 In normal gases, at audible frequencies, the pressure fluctuations occur under essentially adiabatic conditions (no heat is transferred between adjacent gas particles). Speed of sound then becomes: where : 1.4forair and P ρRT (Ideal Gas Law) C P C c v = γ= p = = ρ γ
Head Loss Calculations - Memphis
www.ce.memphis.eduthe mass flow rate is 0.04 slug/s. Assuming ideal gas conditions and relatively constant density in the system, determine the pressure drop in the duct. 43 Head Loss Example ! A rectangular galvanized duct 4 in by 2 ft conveys heated air (T=98°F) to a locker room. The duct is 30 ft long, and the mass flow rate is 0.04 slug/s. Assuming ideal gas
Thermal Expansion Coefficients
galileoandeinstein.physics.virginia.eduAn Ideal Gas Physicists at this point introduce the concept of an “Ideal Gas”. This is like the idea of a frictionless surface: it doesn’t exist in nature, but it is a very handy approximation to some real systems, and makes problems much easier to handle …
C.11Emission Rate Calculations - US EPA
archive.epa.govAnother parameter used in calculations of gases is the Ideal Gas Constant, represented as R. This constant is 0.06236 (mm Hg)(m3)/(g-mole)(°K) ... Other stack gas parameters are …
15-3 Constant Volume and Constant Pressure Processes
www.webassign.netA sample of monatomic ideal gas is initially at a temperature of 200 K. The gas occupies a constant volume. Heat is then added to the gas until the temperature reaches 400 K. This process is shown on the P-V diagram in Figure 15.8, where the system moves from state 1 to state 2 by the process indicated. The
Chapter 8: Gas Power Cycles - Saylor Academy
resources.saylor.orgideal gas. • All the processes that make up the cycle are internally reversible. • The combustion process is replaced by a heat-addition process from an external source. • A heat rejection process that restores the working fluid to its initial state replaces the exhaust process.
HYDROGEN GAS EVOLUTION AND VENTILATION FROM …
www.mathscinotes.comIn ventilation calculations it is required that one know the weight of a particular volume of hydrogen. This conversion is made using the Ideal Gas Law, 2. Grams Hydrogen = 2PV/(82.06*T), P=pressure, atmospheres V=hydrogen volume, cc T= absolute temperature, K M=mass of hydrogen, grams To probe into the intricacies of simple hydrogen
Technology Assessment of a Fuel Cell Vehicle: 2017 Toyota ...
publications.anl.govIdeal gas law Possible. Gravimetric ... ±0.25% gas flow accuracy; 0.0002 g/cc density accuracy The output of the meters is 4-20mA. The output on both meters is scaled from 4-20mA to 0-3gr/sec in the data acquisition system ... AIR COMPRESSOR POWER CALCULATIONS.
Bonus * Bonus * Bonus Infinitesimal work done dw …
www.columbia.eduWork done is NOT independent of path : Change the State of a gas two different ways: Consider n moles of an ideal gas w = 0 for 2nd step since V = const
Thermodynamic Properties and calculation
web.iit.eduAssume air to be an ideal gas with the constant heat capacities, C V = (5/2)R and C P = (7/2)R. Calculate the work required, heat transferred, and the changes in internal energy and enthalpy …
Chapter 18: Solutions - Materials Science
www.mse.berkeley.eduNotes on the Thermodynamics of Solids J.W. Morris, Jr.; Fall, 2008 Page 398 The entropy of a one-component ideal gas can be found by integrating the thermal
Ideal Gas Property Tables
www.egr.msu.eduIdeal Gas Property Tables in SI Units for ME 201 Section 001 Spring 2012 Craig W. Somerton Asscoiate Professor Department of Mechanical Engineeirng Michigan State University East Lansing, MI 48824 somerton@egr.msu.edu