Search results with tag "Mdpi"
Business Terms and Conditions of MDPI AG
www.mdpi.comFurthermore, MDPI may grant discounts and waivers in exceptional cases, however, reserves the right to decline such discounts and waivers without specifying a reason. 4.3 Payments to MDPI are due within 5 days of sending the invoice to the Customer. Longer payment terms up to a maximum of 60 days can be granted by MDPI in written form by e-mail on
粗悪オープンアクセス(OA ジャーナルについて
www.ripo.ynu.ac.jpDec 06, 2018 · →中国・スイスに本部を置くMDPI社の例;” MDPI is very much a hit-and-miss.”) ※MDPIのケース:当初はハゲタカジャーナルと名指しされリストに掲載 されていたものの、現在はOA. 学術出版社協会に加盟し、リスト上でも雑誌
an Open Access Journal by MDPI
www.mdpi.coman Open Access Journal by MDPI ... EMERGING SOURCES CITATION INDEX. Author Benefits Editor-in-Chief Prof. Dr. Mehrdad Massoudi Message from the Editor-in-Chief Fluids (ISSN 2311-5521) is an international journal on all aspects of fluids in open access format: research
CS224W: Machine Learning with Graphs Jure Leskovec, http ...
web.stanford.eduImage credit: MDPI Scene Graphs Image credit: math.hws.edu Regulatory Networks Image credit: ese.wustl.edu Main question: How do we take advantage of relational structure for better prediction? 9/22/2021
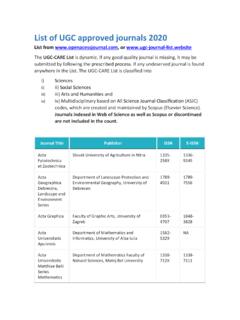
List of ugc approved journals 2020 - Tata Institute of ...
www.tiss.eduBioengineering MDPI 2306-5354 NA Biological Theory Springer 1555-5542 1555-5550 . Biotechnology Research and Innovation Elsevier 2452-0721 NA Biotecnologia Vegetal Instituto De Biotecnologia De Las Plantas 1609-1841 2074-8647 Blde University Journal of Health Sciences Wolters Kluwer- Medknow ...
Pragmatism as a Research Paradigm and Its ... - MDPI
res.mdpi.comthat are open to empirical inquiry (Creswell and Clark2011). Pragmatist scholars have o ered their particular opinion that there is an objective reality that exists apart from human experience. However, this reality is grounded in the environment and …
CTD STRUCTURE: FROM IND TO NDA
cdn.ymaws.comAn Investigational Medicinal Product Dossier (IMPD) is required for approval of clinical trials by competent authorities in the European Union (EU)—the IMPD follows CTD structure + Paediatric investigation plan (PIP) may be required by European Medicines Agency (EMA) + Can be preceded by a request for Scientific Advice from EMA
EU GMP Requirements
www.ema.europa.euIMPD = Investigational Medicinal Product Dossier (part of CTA) . PSF = Product Specification File (references for manufact.) . Comparator = reference product (active or placebo) . Randomisation = assigning trial subjects to treatment or control groups by using an element of chance . Blinding = keeping parties unaware of treatment assignment. EMEA
Philippines: Maritime Industry Development Plan (MIDP ...
marina.gov.phPDP Philippine Development Plan PFDA Philippine Fisheries Development PFSP Plan Port Facility Security Plan PHIVIDEC Philippine Veterans Investment Development Corporation . THE PHILIPPINE MARITIME INDUSTRY DEVELOPMENT PLAN 2019-2028 Page xi PIA Philippine Information Agency PMMA Philippine Merchant Marine Academy ...
Guideline on the requirements for quality documentation ...
www.ema.europa.euThe investigational medicinal product dossier ( IMPD) should be provided in a clearly structured format following the CTD format of Module 3 and include the most up-to-date available information relevant to the clinical trial at time of submission of the clinical trial application.
Strategies for CMC Development and Manufacturing …
www.cilatus.comStrategy Development and Strategy Execution for Technical (CMC) Development and Manufacturing from Pre-clinical to Commercial Manufacturing Technical (CMC) Development from Discovery to Pre-clinical, to IND/IMPD submission, to Early and Late Stage Clinical, to
株式会社 三越伊勢丹プロパティ ... - impd.co.jp
www.impd.co.jp㈱伊勢丹の関連会社として、 丹進㈱(クリーニング事業)を 設立 その後㈱伊勢丹クリーニングに 社名変更
Upload Essential Documents For Form 44
www.cdsco.nic.inCMC DATA 13. Investigational Medicinal Products Dossier (IMPD) [The IMPD Dossier needs to be prepared as per the prescribed format in reference to the Appendix I of
Principles of Metered-Dose Inhaler Design
www.rcjournal.comPrinciples of Metered-Dose Inhaler Design Stephen P Newman PhD Introduction: Development of the Pressurized Metered-Dose Inhaler Component Parts of the pMDI
Thomas Sudhop, MD - AGAH
www.agah.eu1 What is required for first into man? The EU IMPD Thomas Sudhop, MD 2 Scope Structure and content of an IMPD What is required for first into man trial?
INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER FOR …
cdsco.nic.inC:\Users\website\Desktop\pendency july\IMPD Template Quality.doc Last printed 8/18/2016 8:02:00 PM INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER FOR
Similar queries
MDPI, An Open Access Journal by MDPI, EMERGING SOURCES CITATION INDEX, List of ugc approved journals 2020, Pragmatism, Open, Investigational Medicinal Product Dossier, IMPD, EU GMP Requirements, Product, Philippines: Maritime Industry Development Plan MIDP, PDP Philippine Development Plan, Philippine, Development, Plan, DEVELOPMENT PLAN, The investigational medicinal product dossier, Upload Essential Documents For Form 44, Dossier, IMPD Dossier, Principles of Metered-Dose Inhaler Design, PMDI, Thomas Sudhop, MD, IMPD Thomas Sudhop, MD, INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER FOR