Search results with tag "Meningococcal"
Guidance for public health management of meningococcal ...
assets.publishing.service.gov.uk2.2 Meningococcal group W 8 2.3 Meningococcal group Y 9 2.4 Meningococcal group B 9 2.5 Other meningococcal groups 9. 3. Vaccination programmes. 10 3.1 MenC vaccines 10 3.2 Quadrivalent MenACWY vaccines 10 3.3 MenB vaccine 11. 4. Previous guidance. 12. 5. Pre-admission management. 14 5.1 Recommendation 14. 6. Laboratory investigation of ...
VIS Document Type Description / Concept Name Edition Date ...
www.cdc.govMeningococcal B VIS 8/14/2015 253088698300030111150814 0886983000301 Historic 8/9/2016 Serogroup B Meningococcal Vaccine VIS 8/9/2016 253088698300030111160809 0886983000301 Historic 8/15/2019 Meningococcal B Vaccine VIS 8/15/2019 253088698300030111190815 0886983000301 Historic 8/6/2021
[Type text] Meningococcal vaccines - NCIRS
ncirs.org.auMeningococcal disease is a rare but serious infection caused by the bacterium . Neisseria meningitidis (N. meningitidis). There are 13 serogroups. ... polysaccharides of the organism’s outer membrane capsule. Globally, most cases of meningococcal disease are caused by serogroups A, B, C, W and Y.
Dr. Sear’s Alternative Vaccine Schedule for
mymission.lamission.edu1112yrs Tdap, Meningococcal, HPV (3 doses) 16 Meningococcal Parent’s Notes on each Vaccine HIB Pneumococcal (Pc) DTaP (Dephtheria, Tetanus, and Pertussis) Hep B Polio MMR Chickenpox Hep A Flu Meningococcal HPV Combination Vaccines
07.280 Meningococcal Conjugate (Group C) Vaccine ...
albertahealthservices.ca• Outbreak control of laboratory-confirmed serogroup C invasive meningococcal disease in consultation with zone Medical Officers of Health For disease information, contact assessment and reporting guidelines refer to . Public Health Notifiable Disease Management Guidelines – Meningococcal Disease, Invasive.
Many vaccine information statements are Meningococcal B ...
www.immunize.orgS Meningococcal B Vaccine ONLY 42 ..C. 3aa26 862021 Vaccine Information Statement 3. Talk with your health care provider Tell your vaccination provider if the person getting the vaccine: Has had an allergic reaction after a previous dose of meningococcal B vaccine, or has any severe, life-threatening allergies Is pregnant or breastfeeding
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
www.ema.europa.euMeningococcal Infection Due to its mechanism of action, the use of Soliris increases the patient’s susceptibility to meningococcal infection (Neisseria meningitidis). Meningococcal disease due to any serogroup may occur. To reduce the risk of infection, all patients must be vaccinated at least 2 weeks prior to receiving Soliris unless the risk
Communicable Disease Control Manual Chapter 2 ...
www.bccdc.caMen-C -C Meningococcal serogroup C conjugate vaccine Men-C -ACYW -135 Meningococcal quadrivalent conjugate vaccines (serogroups A, C, Y, W -135) MMR Measles, mumps and rubella v accine MMRV Measles, mumps, rubella and varicella vaccine PCV13 Pneumococcal conjugate vaccine, 13 -valent vaccine
Serogroup W-135 Meningococcal Disease during the Hajj, 2000
wwwnc.cdc.govAn outbreak of serogroup W-135 meningococcal dis-ease occurred during the 2000 Hajj in Saudi Arabia. Disease was reported worldwide in Hajj pilgrims and their
Mening Serogroup - Centers for Disease Control and …
www.cdc.govserogroup B meningococcal disease, including: People at risk because of a serogroup B meningococcal disease outbreak Anyone whose spleen is damaged or has been removed, including people with sickle cell disease Anyone with a rare immune system condition called “complement component deficiency”
1. Why get vaccinated? 2. Meningococcal ACWY vaccine
www.cdc.govIC S Meningococcal ACWY Vaccine ONLY 42 ..C. 3aa26 62021 Vaccine Information Statement 3. Talk with your health care provider Tell your vaccination provider if the person getting
Commonly Administered Pediatric Vaccines - CCHAP
cchap.orgJan 01, 2016 · Meningococcal polysaccharide vaccine, serogroups A, C, Y, W-135, quadrivalent (MenACWY or MPSV4), for subcutaneous use sanofi pasteur Menomune 1 90734 Meningococcal conjugate vaccine, serogroups A, C, Y and W-135 quadrivalent (MenACWY or MCV4) , for IM use sanofi pasteur Novartis Menactra Menveo 1
1. Why get vaccinated? 2. Meningococcal ACWY vaccine
www.immunize.orgIC S Meningococcal ACWY Vaccine ONLY 42 ..C. 3aa26 62021 Vaccine Information Statement 3. Talk with your health care provider Tell your vaccination provider if the person getting
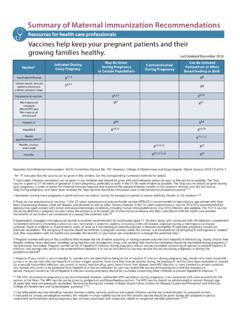
ACOG-Summary of Maternal Immunization Recommendations
www.cdc.gov7. Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. Centers for Disease Control
Iowa Department of Public Health Certificate of Immunization
iris.iowa.govtype B type B 1 1 dose if received when the applicant is 15 months of age or older. Hib vaccine is not required for persons 60 months of age or older. 7 Polio vaccine is not required for persons 18 years of age or older. Meningococcal (A, C, W, Y) 1 dose of meningococcal vaccine received on or after 10 years of age for the applicant in
University Health Services Mānoa - hawaii.edu
www.hawaii.edu#1 #2 #3 Human Papillomavirus (HPV) #1 #2 #3 Meningococcal Quadrivalent #1 Serogroup B Meningococcal (MenB) #1 #2 #3 (if needed) Polio
School meningococcal vaccine requirements
www.health.ny.govor older, then a second dose will not be required. The NYS school immunization requirements allow for a grace period of up to 4 days before the 16th birthday for receipt of the dose. A dose of vaccine received 5 or more days before the 16th birthday will not meet the 12 th grade meningococcal vaccine requirement.
VACCINES FOR CHILDREN (VFC) IMMUNIZATION SERVICE …
oeps.wv.gov90620 Meningococcal recombinant protein and outer membrane vesicle vaccine, serogroup B, 2 dose schedule, for intramuscular 90621 Meningococcal recombinant lipoprotein vaccine, serogroup B, 2 or 3 dose schedule, for ... 90734 Meningicoccal conjugate vaccine, serogoups A, C, Y and W-135 (quadtivalent), for intramuscular use (Menactra).
Personal Beliefs Exemption Form Kindergarten 12 Grade Only
azdhs.govI Meningococcal: have been informed thatby not receiving this vaccine, my child may be at increased risk of developing meningococcal disease. Serious symptoms and effects of this disease include: brain damage, sepsis (systemic infection) permanent scarring …
Upload documents to your Patient Access Portal
www.hawaii.eduMENINGOCOCCAL CONJUGATE VACCINE (A, C, Y, W-135) isd require for first-year college students living in on-campus housing who are age 16 through 21. You will not be allowed to check into your on-campus ... Hepatitis A & B 2. Serogroup B Meningococcal (MenB) 3. Polio 4. HPV
ARKANSAS STATE BOARD OF HEALTH - Arkansas …
www.healthy.arkansas.govA, meningococcal disease, and varicella (chickenpox) (See Table II.), as evidenced by an immunization record from a licensed physician or a public health department acknowledging the immunization. 2. The requirements for entry into school are: Kindergarten: At least four doses of Diphtheria/Tetanus/Acellular Pertussis (DTaP),
DEPARTMENT OF PUBLIC HEALTH - Connecticut
portal.ct.govor verification of disease. 28 days between doses is acceptable if the doses have already been administered. Meningococcal: 1 dose • DTaP vaccine is not administered on or after the 7. th. birthday. • Tdap can be given in lieu of Td vaccine for children 7 …
SCHOOL & DAY CARE MINIMUM IMMUNIZATION …
www.vdh.virginia.govrequired for children 7 years of age and older who do not meet the minimum requirements for tetanus and diphtheria. Effective A booster dose of Tdap vaccine is required for all children entering the 7th grade. Meningococcal Conjugate (MenACWY) Vaccine - Effective July 1, 2021, a minimum of 2 doses of MenACWY vaccine.
Latex in Vaccine Packaging - Centers for Disease Control ...
www.cdc.govAppendix B Appendix B-11 B Latex in Vaccine Packaging “Immediate-type allergic reactions due to latex allergy have been described after vaccination, but such reactions ... MenB = serogroup B meningococcal vaccine; MMR = measles, mumps, and rubella; MMRV = …
Reportable Diseases/Conditions in Florida
www.floridahealth.govMeningococcal disease ... or any laboratory licensed under chapter 483 that diagnoses or suspects the existence of a disease of public health significance shall immediately report the fact to the Department of Health.” Florida’s county health departments serve a s the Department’s representative in this reporting
2021 Recommended Immunizations for Children from 7 …
www.cdc.gova serogroup B meningococcal (MenB) vaccine. These shaded boxes indicate when the vaccine is recommended for all children unless your doctor tells you that your child cannot safely receive the vaccine. These shaded boxes indicate the vaccine should be given if a child is catching up on missed vaccines.
Arizona Administrative Code Requires Providers to: Report ...
www.azdhs.govCysticercosis Meningococcal invasive disease Typhus fever Dengue Mumps Vaccinia-related adverse event O Diarrhea, nausea, or vomiting Novel coronavirus infection (e.g., SARS or MERS) Vancomycin-resistant or Vancomycin-intermediate Staphylococcus aureus
Infection Control in Healthcare Personnel: Epidemiology ...
www.cdc.govNov 05, 2021 · Infection Control in Healthcare Personnel: Epidemiology and Control of Selected Infections Transmitted Among Healthcare Personnel and Patients . Diphtheria, Group A Streptococcus, Meningococcal Disease, and Pertussis Sections . Centers for Disease Control and Prevention . National Center for Emerging and Zoonotic Infectious Diseases
Appendix A: Disease-Specific Chapters
www.health.gov.on.ca2 Meningococcal disease, invasive Communicable Virulent Health Protection and Promotion Act: Ontario Regulation 558/91 – Specification of Communicable Diseases
FOR SCHOOLS AND PARENTS: K-12 IMMUNIZATION …
nj.govMeningococcal (MenACWY) vaccines administered at age 10 or older will be accepted for NJ school attendance.As of the 2020-2021 school year, children who receive a Tdap before age 10 would need to receive an additional dose to meet NJ’s immunization requirements for …
Important! Do Not Delay!
studenthealth.ucf.eduMeningococcal B Serogroup (Bexsero/Trumenba) Covid -19 (Johnson & Johnson , Moderna, Pfizer) DO NOT WRITE HERE / DO NOT WRITE HERE / DO NOT WRITE HERE . An official stamp from a doctor's office, clinic, or Health Department AND an authorized signature must appear on this form or on the official document(s) attached in order to be accepted.
8. Altered Immunocompetence
www.cdc.govMeningococcal serogroup B vaccines are licensed for persons 10-25 years of age and are recommended for persons 10 years of age or older for persons with high-risk conditions like functional or anatomic asplenia or persistent complement component deficiency. There are presently no recommendations for booster doses of either MenB
2021 Childhood Vaccine School-Requirements
www.vdh.virginia.govWhat are the changes regarding 7th-grade school entry vaccination requirements? Children entering 7th grade are required to present proof of their first dose of the human papillomavirus (HPV) vaccine, one booster of the tetanus, diphtheria and pertussis vaccine (Tdap), and their first dose of the meningococcal conjugate vaccine (MenACWY).
Vaccinations for Adults -- You're never too old to get ...
www.immunize.orgGetting vaccinated is a lifelong, life-protecting job. Don’t leave your healthcare provider’s office without making sure you’ve had all the vaccinations you need. Hepatitis A (HepA) Measles, mumps, rubella (MMR) Meningococcal ACWY (MenACWY) Tetanus, diph- theria, whooping cough (pertussis) ( Tdap, d) Varicella (Chickenpox) Zoster ...
Vaccine Requirements For Children Enrolled in Preschool ...
health.maryland.govRequirements for the 2020-21 school year are: 2 doses of Varicella vaccine for entry into Kindergarten, 1st, 2nd , 3rd , 4th , 5th AND 6th grades 1 dose of Tdap vaccine for entry into 7th, 8th, 9th, 10th, 11thAND 12th grades 1 dose of Meningococcal (MCV4) vaccine for entry into 7th, 8th, 9th, 10th,11thAND 12th grades
Vaccine Requirements For Children Enrolled in Preschool ...
health.maryland.govRequirements for the 2021-22 school year are: 2 doses of Varicella vaccine for entry into Kindergarten, 1st, 2nd , 3rd , 4th , 5th, 6thAND 7th grades 1 dose of Tdap vaccine for entry into 7th, 8th, 9th, 10th, 11thAND 12th grades 1 dose of Meningococcal (MCV4) vaccine for entry into 7th, 8th, 9th, 10th,11thAND 12th grades
Prevention and Control of Meningococcal Disease
www.cdc.govRecommendations and Reports. 2 MMWR / March 22, 2013 / Vol. 62 / No. 2. This report is a comprehensive summary of previously published . recommendations (Box 1) …
Meningococcal vaccines for Australians - NCIRS
ncirs.org.auMeningococcal vaccines for Australians | 1 July 2020 Fact sheet 3 . The disease . Meningococcal disease is a relatively rare but serious infection caused by the bacterium . Neisseria meningitidis, commonly known as the meningococcus. There are 13 serogroups, distinguished by differences in the surface polysaccharides of the organism’s outer ...
Meningococcal Vaccination Response Form - farmingdale.edu
www.farmingdale.eduCheck one box and sign below. I have (for students under the age of 18: My child has): o had meningococcal immunization within the past 5 years. The vaccine record is attached. Note: The Advisory Committee on Immunization Practices recommends that all first-year college students up to age 21 years should have at
Meningococcal ACWY Vaccine Recommendations by Age …
www.immunize.orgTravelers to or residents of countries where meningococcal disease is hyperendemic or epidemic, people present during outbreaks caused by a vaccine serogroup,2 and other people with prolonged increased risk for exposure (e.g., microbiologists routinely working with Neisseria meningitidis). For age 2 through 6 months
Meningococcal ACWY Vaccine Recommendations by Age and …
immunize.orgMeningococcal ACWY . V accine Recommendations by Age an d Risk F actor . footnotes. 1. 5.The minimum interval between doses of MenACWY is 8 . weeks.
Meningococcal Conjugate Vaccines (MCV4)
dph.georgia.gov• MPSV4 (Meningococcal Polysaccharide Vaccine), while approved for persons age 2 years & older, should only be used for persons age 56 years & older or when a contraindication to MCV4 (but not MPSV4) exists • Persons who inadvertently receive MPSV4 should be revaccinated with MCV4 using a minimum interval of 8 weeks
Similar queries
Guidance for public health management of meningococcal, Meningococcal, Meningococcal B, Serogroup B meningococcal, Type text] Meningococcal vaccines, Vaccine, Control, Guidelines, Management Guidelines, S Meningococcal, SUMMARY OF PRODUCT CHARACTERISTICS, Serogroup, Meningococcal serogroup, 135 Meningococcal, Serogroup W-135 meningococcal, Hajj, Centers for Disease Control and, Meningococcal disease, Sickle cell disease, Immunization, Disease, Certificate of Immunization, School meningococcal vaccine requirements, School, Grace, Exemption, Arkansas, Requirements, Latex in Vaccine Packaging, B Latex in Vaccine Packaging, Reportable Diseases, And Control, Prevention, Appendix A: Disease-Specific Chapters, Meningococcal B Serogroup, 2021 Childhood Vaccine School-Requirements, Vaccinations for Adults, Vaccine Requirements, Prevention and Control of Meningococcal Disease


![[Type text] Meningococcal vaccines - NCIRS](/cache/preview/0/e/3/6/6/1/8/a/thumb-0e36618a7370d9cfa86ed8d2bb37b564.jpg)