Search results with tag "Preferred reporting items for systematic"
PRISMA-P (Preferred Reporting Items for Systematic review ...
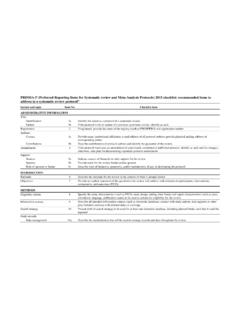
prisma-statement.orgPRISMA-P (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol* Section and topic Item No Checklist item ADMINISTRATIVE INFORMATION Title: Identification 1a Identify the report as a protocol of a systematic review Update 1b If the protocol is ...
AMSTAR 2: a critical appraisal tool for systematic reviews ...
www.bmj.comreviews, which led to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.3 The reporting guide for systematic reviews of observational (non-randomised) studies is MOOSE (Meta-analysis of Observational Studies in Epidemiology).4 The quality of reporting of a systematic
U.S. Medical Eligibility Criteria for Contraceptive Use, 2016
www.cdc.gov[CHCs]). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for reporting systematic reviews (8, 9), and strength and quality of the evidence were assigned using the system of the U.S. …
The PRISMA Statement for Reporting Systematic Reviews …
www.prisma-statement.orgthe reporting guidance to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses). Development of PRISMA The PRISMA Statement was developed by a group of 29 review authors, methodologists, clinicians, medical editors, and consum-ers [12]. They attended a three-day meeting in 2005 and
PRISMA 2009 checklist
www.prisma-statement.orgFunding 27 Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097.
Writing a Systematic Literature Review: Resources for ...
apsu.org.au2 Writing a Systematic Literature Review: Resources for Students and Trainees Some key resources are highlighted in the next few pages – researchers around the world have found these useful – it’s worth a look and it might save you a lot of time! PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement ...
How to Write a Systematic Review: A Step-by-Step Guide
www.upoj.orgreviews are the PRISmA statement (Preferred Reporting Items for Systematic reviews and meta Analyses).3These guidelines facilitate the reporting of appropriate information (Figure 1). Conducting and Reviewing the Search Once a justified study question and detailed study protocol are in place, the systematic review process can proceed.
The PRISMA 2020 statement: an updated guideline for ...
www.bmj.comThe Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have
Undertaking a Systematic Review: What You Need to Know
www.nihlibrary.nih.gov• PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) • PRISMA-E (PRISMA + health equity reporting) • MOOSE (Meta-analysis of Observational Studies in Epidemiology) • RAMESES publication standards: meta-narrative reviews • EQUATOR • Collects guidance documents on reporting SRs and other types of health research
PRISMA 2020 explanation and elaboration: updated guidance ...
www.bmj.comreview findings. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement was developed to facilitate transparent and complete reporting of systematic reviews and has been updated (to PRISMA 2020) to reflect recent advances in systematic review methodology and terminology. Here, we present the explanation and
メタアナリシス
www.nshi.jpHandbook for Systematic Reviews of Interventions) •Cochrane Collaborationによる非常に有 な解説書(コクラン・レビュー実施ガイド) •PRISMA声明(Preferred Reporting Items for Systematic Reviews and Meta-Analyses) •報告のためのガイドライン(CONSORTのメ タアナリシス版) 16 実施方法学習に有用 ...
The PRISMA 2020 statement: an updated guideline for ...
hbg.cochrane.orgBackground: The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did and what they found. Over the last decade, there have
Drug treatment for panic disorder with or without ...
www.bmj.comalso undertook a network meta-analysis to compare individual SSRIs (eg, fluoxetine, fluvoxamine, paroxetine, sertraline, escitalopram, and citalopram). Methods This systematic review and network meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA).11 12
PEDOMAN PENULISAN TUGAS AKHIR - FAKULTAS …
fk.ub.ac.idUntuk menentukan protokol SLR dapat menggunakan metode PRISMA (Preferred Reporting Items For Systematic Reviews and Meta Analyses). Tahapan dalam PRISMA untuk melakukan SLR a. Mendefinisikan kriteria kelayakan (Inclusive & Exclusive Criteria) b. Mendefinisikan sumber informasi (Electronic Database) c. Pemilihan Literatur (Study Selection) d.
Preferred reporting items for systematic review and meta ...
prisma-statement.orgPRISMA-P: Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols A guideline to help authors prepare protocols for planned systematic reviews and meta-analyses that provides them with a minimum set of items to be included in …
Preferred reporting items for systematic review and meta ...
prisma-statement.orgPreferredreportingitemsforsystematicreviewand meta-analysisprotocols(PRISMA-P)2015:elaboration andexplanation LarissaShamseer1,DavidMoher1,MikeClarke2,DavinaGhersi3 ...
Systematic and Non-Systematic Literature Review ...
www.evidera.comMoher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Steward LA, and PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 Statement.
Similar queries
Preferred Reporting Items for Systematic, Checklist, Items, Systematic, For Systematic, Reporting, PRISMA, Reporting Systematic, Review, Systematic review, Writing a Systematic Literature Review: Resources for, Writing a Systematic Literature Review: Resources for Students, And Meta, To Write a Systematic Review: A Step-by, Meta, PEDOMAN PENULISAN TUGAS AKHIR, Systematic and Non-Systematic Literature