Transcription of CPT® 2016 Code Changes - Medtron Software
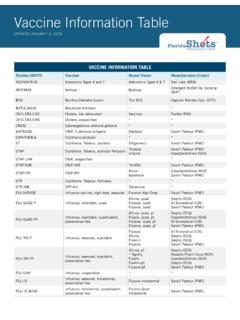
1 20152016 CodeDescriptorChangeCrosswalkCodeDescrip torAdvice90632 Hepatitis A vaccine (HepA), adultdosage, for intramuscular useRevised--Hepatitis A vaccine (HepA), adult dosage,for intramuscular useCPT 2016 changesthe wording of theofficial descriptor, orOD, for code 90632to include theabbreviation "(HepA)" after thename of the A vaccine (HepA),pediatric/adolescent dosage-2dose schedule, for intramuscularuseRevised--Hepatitis A vaccine (HepA),pediatric/adolescentdosage -2 doseschedule, forintramuscular useCPT 2016 changesthe wording of theofficial descriptor, orOD, for code 90633to include theabbreviation "(HepA)" after thename of the A vaccine (HepA),pediatric/adolescent dosage-3dose schedule, for intramuscularuseRevised--Hepatitis A vaccine (HepA),pediatric/adolescentdosage -3 doseschedule, forintramuscular useCPT 2016 changesthe wording of theofficial descriptor, orOD, for code 90634to include theabbreviation "(HepA)" after thename of the conjugatevaccine, serogroups C & Y andHamophilus influenza type bvaccine (Hib-MenCY), 4 doseschedule, when administered tochildren 2-18 months of age, forintramuscular useRevised--Meningococcalconjugate vaccine ,serogroups C & Y andHemophilus influenzaBHaemophilusinfluenzae type bvaccine (Hib-MenCY),4 dose schedule,when administered tochildren 2-1518months of age, forintramuscular useCPT 2016 changesthe wording of theofficial descriptor, orOD, for code 90644from "2-15 months ofage" to "2-18 monthsof age.
2 " CPT doesnot provide anindication as to thereason for thischange in influenza b vaccine (Hib), HbOC conjugate (4 doseschedule), for intramuscular useDeletedThe AMAdoes notprovidecrosswalkcodes forthisdeletedcode The 2016 code setdeleted 90645 as anobsolete influenza b vaccine (Hib), PRP-D conjugate, forbooster use only, intramuscularuseDeletedThe AMAdoes notprovidecrosswalkcodes forthisdeletedcode The 2016 code setdeleted 90645 as anobsolete vaccinecode. 90647 Hamophilus influenza type bvaccine (Hib), PRP-OMPconjugate, 3 dose schedule, forintramuscular useRevised--HemophilusinfluenzaHaemophil usinfluenzae type bvaccine (Hib), PRP-OMP conjugate (, 3dose schedule), forintramuscular useCPT 2016 removesthe parentheses fromaround "3-doseschedule" in theofficial descriptor, orOD, for code90647. CPT doesnot provide anindication as to thereason for thischange, but it couldsimply be to makethe OD consistentwith CPT style forthe use this codewhen the provideradministers a form ofthe vaccine thatprotects againstHemophilusinfluenza B, bacteriathat causesmeningitis, a seriousdisease that attacksthe meninges, thecovering of the brainand spinal cord.
3 Heinjects the vaccineinto a muscle as partof a influenza type bvaccine (Hib), PRP-T conjugate,4 dose schedule, forintramuscular useRevised--HemophilusinfluenzaHaemophil usinfluenzae type bvaccine (Hib), PRP-Tconjugate (, 4 doseschedule), forintramuscular useCPT 2016 removesthe parentheses fromaround "3-doseschedule" in theofficial descriptor, orOD, for code90648. CPT doesnot provide anindication as to thereason for thischange, but it couldsimply be to makethe OD consistentwith CPT style forthe use this codewhen the provideradministers a form ofthe vaccine thatprotects againstHemophilusinfluenza B, bacteriathat causesmeningitis, a seriousdisease that attacksthe meninges, thecovering of the brainand spinal cord. Heinjects the vaccineinto a muscle as partof a Papillomavirus vaccine ,types 6, 11, 16, 18, quadrivalent(4vHPV), 3 dose schedule, forintramuscular useRevised--Human Papillomavirus(HPV)Papillomavirusvaccine , types 6, 11,16, 18 (quadrivalent),quadrivalent (4vHPV),3 dose schedule, forintramuscular useCPT 2016 removesthe abbreviation "(HPV)" and theparentheses fromaround "quadrivalent"and adds "(4vHPV)"after the wordquadrivalent in theofficial descriptor, orOD, for code 90649,which is basically astyle this codewhen the providerinjects an alteredform of the humanpapilloma virus, orHPV, into a muscle toprovide immunity tofour types of HPV, acause of genitalwarts and cervicalcancer.
4 Headministers thevaccine as part of athree-dose Papillomavirus vaccine ,types 16, 18, bivalent (2vHPV), 3dose schedule, for intramuscularuseRevised--Human Papillomavirus(HPV)Papillomavirusvaccine , types 16, 18,bivalent (2vHPV), 3dose schedule, forintramuscular useCPT 2016 removes"(HPV)" following thename of the vaccineand adds "(2vHPV)"after bivalent in theofficial descriptor Papillomavirus vaccine ,types 6, 11, 16, 18, 31, 33, 45,52, 58, nonavalent (9vHPV), 3dose schedule, for intramuscularuseRevised--HumanPapillomav irusvaccine types 6, 11,16, 18, 31, 33, 45, 52,58, nonavalent(HPV)(9vHPV), 3dose schedule, forintramuscular useCPT 2016 replaces"(HPV)" afternonvalent with "(ovHPV)" in theofficial descriptor vaccine , inactivated(IIV), subunit, adjuvanted, forintramuscular useRevised--Influenza vaccine ,inactivated (IIV),subunit, adjuvanted,for intramuscular useCPT 2016 adds "(IIV)" after "Influenzavaccine, inactivated"in the officialdescriptor for virus vaccine , trivalent(IIV3), split virus, preservativefree, when administered tochildren 6-35 months of age, forintramuscular useRevised--Influenza virusvaccine, trivalent(IIV3), split virus,preservative free,when administered tochildren 6-35 monthsof age, forintramuscular useCPT 2016 adds "(IIV3)" after"Influenza virusvaccine, trivalent" inthe official descriptorfor virus vaccine , trivalent(IIV3), split virus, preservativefree, when administered toindividuals 3 years and older, forintramuscular useRevised--Influenza virusvaccine, trivalent(IIV3)
5 , split virus,preservative free,when administered toindividuals 3 yearsand older, forintramuscular useCPT 2016 adds "(IIV3)" after"Influenza virusvaccine, trivalent" inthe official descriptorfor virus vaccine , trivalent(IIV3), split virus, whenadministered to children 6-35months of age, for intramuscularuseRevised--Influenza virusvaccine, trivalent(IIV3), split virus,when administered tochildren 6-35 monthsof age, forintramuscular useCPT 2016 adds "(IIV3)" after"Influenza virusvaccine, trivalent" inthe official descriptorfor virus vaccine , trivalent(IIV3), split virus whenadministered to individuals 3years of age and older, forintramuscular useRevised--Influenza virusvaccine, trivalent(IIV3), split virus,when administered toindividuals 3 years ofage and older, forintramuscular useCPT 2016 adds "(IIV3)" after"Influenza virusvaccine, trivalent" inthe official descriptorfor virus vaccine , trivalent,live (LAIV3), for intranasal useRevised--Influenza virusvaccine, trivalent, live(LAIV3), for intranasaluseCPT 2016 adds "(LAIV3)" after"Influenza virusvaccine, trivalent,live" in the officialdescriptor for virus vaccine (ccIIV3),derived from cell cultures,subunit, preservative andantibiotic free, for intramuscularuseRevised--Influenza virusvaccine (ccIIV3),derived from cellcultures, subunit,preservative andantibiotic free, forintramuscular useCPT 2016 adds "(ccIIV3)" after"Influenza virusvaccine" in the officialdescriptor for virus vaccine (IIV)
6 , splitvirus, preservative free,enhanced immunogenicity viaincreased antigen content, forintramuscular useRevised--Influenza virusvaccine (IIV), splitvirus, preservativefree, enhancedimmunogenicity viaincreased antigencontent, forintramuscular useCPT 2016 adds "(IIV)" after "Influenzavirus vaccine " in theofficial descriptor virus vaccine , live(LAIV), pandemic formulation, forintranasal useRevised--Influenza virusvaccine, live (LAIV),pandemic formulation,live, for intranasal useCPT 2016 adds "(LAIV)" after"Influenza virusvaccine, live" in theofficial descriptor virus vaccine (IIV),pandemic formulation, split virus,preservative free, forintramuscular useRevised--Influenza virusvaccine (IIV),pandemic formulation,split virus,preservative free, forintramuscular useCPT 2016 adds "(IIV)" after "Influenzavirus vaccine " in theofficial descriptor virus vaccine (IIV),pandemic formulation, split virus,adjuvanted, for intramuscular useRevised--Influenza virusvaccine (IIV),pandemic formulation,split virus, adjuvanted,for intramuscular useCPT 2016 adds "(IIV)" after "Influenzavirus vaccine " in theofficial descriptor virus vaccine (IIV),pandemic formulation, split virusfor intramuscular useRevised--Influenza virusvaccine (IIV),pandemic formulation,split virus, forintramuscular useCPT 2016 adds "(IIV)" after "Influenzavirus vaccine " in theofficial descriptor conjugatevaccine, 7 valent, forintramuscular useDeletedThe AMAdoes notprovidecrosswalkcodes forthisdeletedcode The 2016 code setdeleted 90669.
7 Atthis time, CPT doesnot provide anindication as to thereason for deletion ofthis code . CPT alsodoes not provide acrosswalk for thiscode but check withthe individual payersfor their policies oncodes to report forthis service. Refer tothe CPT manualalso for furtherdirection as you maybe able to report thisservice or procedureusing an unlistedcode. Remember tosend supportingdocumentation topayers with any claimcontaining anunlisted code . Keepin mind that CPT does not recommendthe use of unlistedprocedures on aregular conjugatevaccine, 13 valent (PCV13), forintramuscular useRevised--Pneumococcalconjugate vaccine , 13valent (PCV13), forintramuscular useCPT 2016 adds "(PCV13)" after"Pneumococcalconjugate vaccine ,13 valent" in theofficial descriptor virus vaccine ,quadrivalent, live (LAIV4), forintranasal useRevised--Influenza virusvaccine, quadrivalent,live (LAIV4), forintranasal useCPT 2016 adds "(LAIV4)" after"Pneumococcalconjugate vaccine ,quadrivalent, live" inthe official descriptorfor virus vaccine , trivalent(RIV3), derived from recombinantDNA, hemagglutinin (HA) proteinonly, preservative and antibioticfree, for intramuscular useRevised--Influenza virusvaccine, trivalent(RIV3), derived fromrecombinant DNA(RIV3), hemagglutinin(HA)
8 Protein only,preservative andantibiotic free, forintramuscular useCPT 2016 moves (RIV3) to follow Influenza virusvaccine, trivalent inthe official descriptorfor vaccine , pentavalent(RV5), 3 dose schedule, live, fororal useRevised--Rotavirus vaccine ,pentavalent (RV5), 3dose schedule, live,for oral useCPT 2016 adds "(RV5)" after"Rotavirus vaccine ,pentavalent,quadrivalent, live" inthe official descriptorfor vaccine , human,attenuated (RV1), 2 doseschedule, live, for oral useRevised--Rotavirus vaccine ,human, attenuated(RV1), 2 doseschedule, live, for oraluseCPT 2016 adds "(RV1)" after"Rotavirus vaccine ,human, attenuated"in the officialdescriptor for virus vaccine ,quadrivalent (IIV4), split virus,preservative free, whenadministered to children 6-35months of age, for intramuscularuseRevised--Influenza virusvaccine, quadrivalent(IIV4), split virus,preservative free,when administered tochildren 6-35 monthsof age, forintramuscular useCPT 2016 adds "(IIV4)" after"Influenza virusvaccine,quadrivalent" in theofficial descriptor virus vaccine ,quadrivalent (IIV4), split virus,preservative free, whenadministered to individuals 3years of age and older, forintramuscular useRevised--Influenza virusvaccine, quadrivalent(IIV4), split virus,preservative free,when administered toindividuals 3 years ofage and older, forintramuscular useCPT 2016 adds "(IIV4)" after"Influenza virusvaccine,quadrivalent" in theofficial descriptor virus vaccine ,quadrivalent (IIV4)
9 , split virus,when administered to children 6-35 months of age, forintramuscular useRevised--Influenza virusvaccine, quadrivalent(IIV4), split virus,when administered tochildren 6-35 monthsof age, forintramuscular useCPT 2016 adds "(IIV4)" after"Influenza virusvaccine,quadrivalent" in theofficial descriptor virus vaccine ,quadrivalent (IIV4), split virus,when administered to individuals3 years of age and older, forintramuscular useRevised--Influenza virusvaccine, quadrivalent(IIV4), split virus,when administered toindividuals 3 years ofage and older, forintramuscular useCPT 2016 adds "(IIV4)" after"Influenza virusvaccine,quadrivalent" in theofficial descriptor vaccine , heat- andphenol-inactivated (H-P), forsubcutaneous or intradermal useDeletedThe AMAdoes notprovidecrosswalkcodes forthisdeletedcode The 2016 code setdeleted 90692 as anobsolete vaccine , acetone-killed,dried (AKD), for subcutaneoususe ( military)Delet