Transcription of Farxiga Prescribing Information
1 US-50977. HIGHLIGHTS OF Prescribing Information ------------------------- DOSAGE FORMS AND STRENGTHS ------------------------ These highlights do not include all the Information needed to use Farxiga Tablets: 5 mg and 10 mg (3). safely and effectively. See full Prescribing Information for Farxiga . ------------------------------ CONTRAINDICATIONS ------------------------------- Farxiga (dapagliflozin) tablets, for oral use Initial Approval: 2014 History of serious hypersensitivity reaction to Farxiga . (4). Patients on dialysis. (4). ----------------------------- RECENT MAJOR CHANGES ----------------------------- Indications and Usage (1) 04/2021 -------------------------- WARNINGS AND PRECAUTIONS -------------------------- Dosage and Administration ( , ) 04/2021 Ketoacidosis in Patients with Diabetes Mellitus: Assess patients who present with signs and symptoms of metabolic acidosis for ketoacidosis regardless of blood Dosage and Administration ( , ) removed 04/2021 glucose level.
2 If suspected, discontinue Farxiga , evaluate and treat promptly. Before Contraindications (4) 04/2021 initiating Farxiga , consider risk factors for ketoacidosis. Patients on Farxiga may Warnings and Precautions ( ) 05/2020 require monitoring and temporary discontinuation of therapy in clinical situations known to predispose to ketoacidosis. ( ). ----------------------------- INDICATIONS AND USAGE ----------------------------- Farxiga is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated for: Volume depletion: Before initiating Farxiga , assess volume status and renal function in the elderly, patients with renal impairment or low systolic blood as an adjunct to diet and exercise to improve glycemic control in adults with pressure, and in patients on diuretics. Monitor for signs and symptoms during type 2 diabetes mellitus. (1) therapy.
3 ( , ). to reduce the risk of hospitalization for heart failure in adults with type 2 diabetes Urosepsis and Pyelonephritis: Evaluate for signs and symptoms of urinary tract mellitus and either established cardiovascular disease or multiple cardiovascular infections and treat promptly, if indicated. ( ). risk factors. (1). Hypoglycemia: Consider a lower dose of insulin or the insulin secretagogue to reduce to reduce the risk of cardiovascular death and hospitalization for heart failure in the risk of hypoglycemia when used in combination with Farxiga . ( ). adults with heart failure with reduced ejection fraction (NYHA class II-IV). (1). Necrotizing Fasciitis of the Perineum (Fournier's Gangrene): Serious, life-threatening to reduce the risk of sustained eGFR decline, end stage kidney disease cases have occurred in patients with diabetes, both females and males.
4 Assess cardiovascular death and hospitalization for heart failure in adults with chronic patients presenting with pain or tenderness, erythema, or swelling in the genital kidney disease at risk of progression. (1) or perineal area, along with fever or malaise. If suspected, institute prompt Limitations of use: treatment. ( ). Not for treatment of type 1 diabetes mellitus. (1) Genital Mycotic Infections: Monitor and treat if indicated. ( ). Farxiga is not recommended for use to improve glycemic control in adults with ------------------------------- ADVERSE REACTIONS ------------------------------- type 2 diabetes mellitus with an eGFR less than 45 mL/ m2. Farxiga is likely to be ineffective in this setting based upon its mechanism of action. (1) The most common adverse reactions associated with Farxiga (5% or greater incidence) were female genital mycotic infections, nasopharyngitis, and urinary Farxiga is not recommended for the treatment of chronic kidney disease in tract infections.
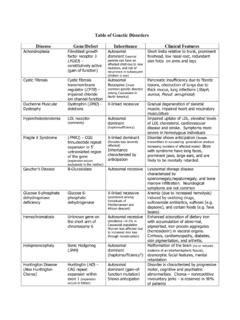
5 ( ). patients with polycystic kidney disease or patients requiring or with a recent history of immunosuppressive therapy for the treatment of kidney disease . To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at Farxiga is not expected to be effective in these populations. (1) 1-800-236-9933 or FDA at 1-800-FDA-1088 or --------------------------- DOSAGE AND ADMINISTRATION ------------------------- -------------------------- USE IN SPECIFIC POPULATIONS -------------------------- Assess volume status and correct volume depletion before initiating. ( ) Pregnancy: Advise females of the potential risk to a fetus especially during the second and third trimesters. ( ). eGFR Recommended Dose Lactation: Farxiga is not recommended when breastfeeding. ( ). (mL/ m2) Geriatrics: Higher incidence of adverse reactions related to hypotension.
6 ( , ). eGFR 45 or greater To improve glycemic control, the recommended Renal Impairment: Higher incidence of adverse reactions related to volume starting dose is 5 mg orally once daily. Dose can be depletion. ( , ). increased to 10 mg orally once daily for additional See 17 for PATIENT COUNSELING Information and Medication Guide. glycemic control. Revised: 04/2021. For all other indications, the recommended starting dose is 10 mg orally once daily. eGFR 25 to less than 45 10 mg orally once daily eGFR less than 25 Initiation is not recommended, however patients may continue 10 mg orally once daily to reduce the risk of eGFR decline, ESKD, CV death and hHF. On dialysis Contraindicated FULL Prescribing Information : CONTENTS* 8 USE IN SPECIFIC POPULATIONS. 1 INDICATIONS AND USAGE Pregnancy Lactation 2 DOSAGE AND ADMINISTRATION. Pediatric Use Prior to Initiation of Farxiga .
7 Geriatric Use Recommended Dosage Renal Impairment 3 DOSAGE FORMS AND STRENGTHS. Hepatic Impairment 4 CONTRAINDICATIONS. 10 OVERDOSAGE. 5 WARNINGS AND PRECAUTIONS. 11 DESCRIPTION. Ketoacidosis in Patients with Diabetes Mellitus 12 CLINICAL PHARMACOLOGY. Volume Depletion Mechanism of Action Urosepsis and Pyelonephritis Pharmacodynamics Hypoglycemia with Concomitant Use with Insulin and Insulin Pharmacokinetics Secretagogues 13 NONCLINICAL TOXICOLOGY. Necrotizing Fasciitis of the Perineum (Fournier's Gangrene). Carcinogenesis, Mutagenesis, Impairment of Fertility Genital Mycotic Infections 14 CLINICAL STUDIES. 6 ADVERSE REACTIONS. Glycemic Control in Patients with Type 2 Diabetes Mellitus Clinical Trials Experience Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus Postmarketing Experience Heart Failure with Reduced Ejection Fraction 7 DRUG INTERACTIONS.
8 Chronic kidney disease Positive Urine Glucose Test 16 HOW SUPPLIED/STORAGE AND HANDLING. Interference with 1,5-anhydroglucitol (1,5-AG) Assay 17 PATIENT COUNSELING Information . * Sections or subsections omitted from the full Prescribing Information are not listed. FULL Prescribing Information Farxiga [see Adverse Reactions ( )]. In placebo-controlled trials of patients with type 1 diabetes mellitus, the risk of ketoacidosis was increased in patients who 1 INDICATIONS AND USAGE received SGLT2 inhibitors compared to patients who received placebo. Fatal cases of Farxiga (dapagliflozin) is indicated: ketoacidosis have been reported in patients taking Farxiga . Farxiga is not indicated for As an adjunct to diet and exercise to improve glycemic control in adults with the treatment of patients with type 1 diabetes mellitus [see Indications and Usage (1)].
9 Type 2 diabetes mellitus. Patients treated with Farxiga who present with signs and symptoms consistent To reduce the risk of hospitalization for heart failure in adults with type 2 diabetes with severe metabolic acidosis should be assessed for ketoacidosis regardless of mellitus and either established cardiovascular disease or multiple cardiovascular presenting blood glucose levels as ketoacidosis associated with Farxiga may be risk factors. present even if blood glucose levels are less than 250 mg/dL. If ketoacidosis is To reduce the risk of cardiovascular death and hospitalization for heart failure in suspected, Farxiga should be discontinued, the patient should be evaluated, and adults with heart failure (NYHA class II-IV) with reduced ejection fraction. prompt treatment should be instituted. Treatment of ketoacidosis may require insulin, To reduce the risk of sustained eGFR decline, end-stage kidney disease , fluid, and carbohydrate replacement.
10 Cardiovascular death, and hospitalization for heart failure in adults with chronic In many of the postmarketing reports, and particularly in patients with type 1 diabetes, kidney disease at risk of progression. the presence of ketoacidosis was not immediately recognized, and the institution of treatment was delayed because the presenting blood glucose levels were below those Limitations of Use typically expected for diabetic ketoacidosis (often less than 250 mg/dL). Signs and Farxiga is not recommended for patients with type 1 diabetes mellitus. It may symptoms at presentation were consistent with dehydration and severe metabolic increase the risk of diabetic ketoacidosis in these patients [see Warnings and acidosis and included nausea, vomiting, abdominal pain, generalized malaise, and Precautions ( )]. shortness of breath. In some but not all cases, factors predisposing to ketoacidosis, Farxiga is not recommended for use to improve glycemic control in adults with such as insulin dose reduction, acute febrile illness, reduced caloric intake, surgery, type 2 diabetes mellitus with an eGFR less than 45 mL/ m2.