Transcription of International Normalized Ratio (INR)
1 The Relationship of the International Normalized Ratio (INR) to the Prothrombin Time (PT) By: William DePond MD, President and Chief Medical Officer MEDLAB In 1983, it was determined that patients receiving long-term anticoagulant therapy may be subject to unnecessary risks of bleeding or thromboembolism because of variability in the commercial thromboplastins used to determine prothrombin time (PT) and consequent uncertainty about the actual intensity of anticoagulation. The accuracy of the PT was noted to be system-dependent showing variations in results due to marked variability in the response of commercial thromboplastin reagents to clotting factors (II, VII, X). This led to the introduction of the International Normalized Ratio (INR), a method developed to normalize the clotting time value by correcting for differences in reagent responsiveness. Thus, laboratory monitoring of oral anticoagulant therapy could be standardized. Each lot of thromboplastin is tested against an International standard and the relationship is expressed as the International Sensitivity Index (ISI).
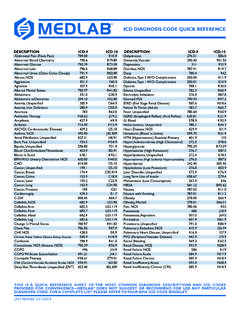
2 The INR uses the ISI to equate all thromboplastins to the reference thromboplastin through the following equation: INR = (patient PT/mean normal PT) ISI. This logarithmic relationship is easily seen in the table below comparing various ISI reagents and the variation of seconds compared to the INR. A patient with an INR of (remember the INRs will be equivalent) will show a PT of seconds using a sensitive ISI reagent ( ) versus seconds an insensitive ISI reagent ( ). PT seconds vs. ISI INR ISI= ISI= ISI= ISI= Reagents with lower ISI values are more responsive to the effects of Warfarin therapy and thus have longer PTs. When a sample from a patient stabilized on Warfarin therapy is tested using two thromboplastin reagents with two different ISIs, the PT (seconds) will be higher with the more sensitive (lower ISI) thromboplastin.
3 Conversely, the PT (seconds) will be lower with the less sensitive (higher ISI) reagent. Nevertheless, the INR values will be equivalent. The College of American Pathologists (CAP) Conference XXXI on Laboratory Monitoring of Anticoagulant Therapy published a recommendation that laboratories use thromboplastins with an ISI between and The use of sensitive thromboplastins is supported by how variations (or errors) in ISI values influence the INR. Variations in ISI can occur due to inherent imprecision in the manufacturer-assigned ISI, changes during transportation or local instrument effect. A thromboplastin reagent with a lower ISI value results in a wider range of PT ratios to obtain a therapeutic INR. This enhances patient safety. Despite the INR system, significant inter-laboratory variation and inaccuracies persist in laboratories which can be can reduce by using a sensitive thromboplastin with an instrument-specific ISI value, determining the geometric mean normal PT for each lot of thromboplastin and ensuring the INR calculation uses the appropriate ISI for reagent lot.
4 If a laboratory uses the PT (seconds) to trigger a critical value call to a clinician, when a change of thromboplastin reagents is made to one with a lower ISI, the laboratory will need to adjust the PT critical value. If a laboratory uses the INR for triggering a critical value call, then a change in thromboplastin reagent would have little impact to critical value reporting. The normal range for the INR for a healthy person is Recommendations for therapeutic levels of anticoagulation based on INR can be found in "Antithrombotic and Thrombolytic Therapy, 8th Ed: ACCP Guidelines", published in the June 2008 (Supplement) issue of Chest. An INR of to is recommended for most indications. The guidelines are helpful but do replace clinical judgment in monitoring patients, as patients vary in their response to oral anticoagulation. Such variations in patient response may be due to intrinsic factors such as genetic factors or extrinsic factors such as medications, disease processes and/or diet.
5 The tables below show some of these factors and their impact on a patient s response to anticoagulation therapy. Cytochrome P450 2C9 and VKORC1 Mutation Analysis Clinical Significance: Warfarin (coumadin) therapy is associated with significant complications because of its narrow therapeutic index and large interpatient dosage variation necessary to achieve an optimal therapeutic response. This variation is due to both genetic and environmental factors. A promoter variant (-1639 G>A) of the Vitamin K epoxide complex subunit 1 (VKORC1) accounts for 25%-44% of this variability and variants of the cytochrome P450 enzyme 2C9 (CYP2C9) account for 10%-15% of this variability. Identification of these Warfarin sensitive variants of the VKORC1 and the CYP2C9 genes may allow a more individualized therapy and reduced risk of bleeding complications. CYP2C9*2 or CYP2C9*3, Metabolize coumarins slowly Twice as likely to have a laboratory or clinical adverse event Reduce Warfarin dosage vitamin K epoxide reductase (VKORC1) Metabolize coumarins slowly Homozygous VKORC1 promoter polymorphism 1639 G>A (aka VKOR 3673, haplotype A, or haplotype*2)
6 Reduce Warfarin dosage compared to genotype GG patients Condition Prothrombin time Partial thromboplastin time Bleeding time Platelet count Vitamin K deficiency or Warfarin prolonged prolonged unaffected unaffected Disseminated intravascular coagulation prolonged prolonged prolonged decreased Von Willebrand disease unaffected prolonged prolonged unaffected Hemophilia unaffected prolonged unaffected unaffected Aspirin unaffected unaffected prolonged unaffected Thrombocytopenia unaffected unaffected prolonged decreased Early Liver failure prolonged unaffected unaffected unaffected End-stage Liver failure prolonged prolonged prolonged decreased Uremia unaffected unaffected prolonged unaffected Congenital afibrinogenemia prolonged prolonged prolonged unaffected Factor V deficiency prolonged prolonged unaffected unaffected Factor X deficiency as seen in amyloid purpura prolonged prolonged unaffected unaffected Glanzmann's thrombasthenia unaffected unaffected prolonged unaffected Bernard-Soulier syndrome unaffected unaffected prolonged decreased Antibiotics Drug Effect on INR Mechanism Comments erythromycin inhibition of metabolism Avoid if possible, otherwise decrease Warfarin by 50% while giving antibiotic and monitor every second day.
7 Metronidazole inhibition of metabolism Avoid if possible, otherwise decrease Warfarin by 50% while giving antibiotic and monitor every second day. quinolones (enoxacin > ciprofloxacin > norfloxacin > nalidixic acid > ofloxacin) inhibition of metabolism; altered protein binding Avoid if possible, otherwise decrease Warfarin by 50% while giving antibiotic and monitor every second day. trimethoprim / sulfamethoxazole inhibition of metabolism; altered protein binding Avoid if possible, otherwise decrease Warfarin by 50% while giving antibiotic and monitor every second day. rifampin induction of metabolism Monitor, dose increase probably required Analgesics Anti-inflammatory Drugs Drug Effect on INR Mechanism Comments phenylbutazone inhibition of metabolism altered protein binding Avoid: anti-platelet effect, increases peptic ulceration aspirin (high dose) direct prolongation of prothrombin time Anti-platelet effect, increases peptic ulceration.
8 Significant risk but low dose aspirin may be justified for some clinical indications. NSAIDs (except phenylbutazone) - minor displacement interactions Cause peptic ulceration, reversible anti-platelet effect, avoid if possible but minimal pharmacokinetic interactions. Lipid Lowering Drugs Drug Effect on INR Mechanism Comments clofibrate unknown Avoid cholestyramine binds Warfarin Colestipol has less effect than cholestyramine Neurological Drugs Drug Effect on INR Mechanism Comments Barbiturates induction of metabolism Potent enzyme inducer, will require a large increase in dose over several weeks carbamazepine induction of metabolism Potent enzyme inducer, will require a large increase in dose over several weeks Gastroenterological Drugs Drug Effect on INR Mechanism Comments cimetidine inhibition of metabolism Avoid: substitute another H2 antagonist Cardiovascular Drugs Drug Effect on INR Mechanism Comments amiodarone inhibition of metabolism May require decrease in dose, consider sotalol or other agent unless extrapyramidal symptoms indicate otherwise.
9 Quinidine unknown Nutritional Supplements Social Drugs Drug Effect on INR Mechanism Comments vitamin K vitamin K Avoid, except when given to correct excess anticoagulation alcohol induction/inhibition of metabolism safe if intake is < 30 Gm/day Miscellaneous Drugs Drug Effect on INR Mechanism Comments disulfuram indirect potentiation Avoid heparin direct prolongation of prothrombin time Prolongation of INR 10-20% if activated partial thromboplastin time is in the therapeutic range antithyroid drugs altered catabolism of clotting factors Increased dose may be required sulfinpyrazone inhibition of metabolism altered protein binding anti-platelet effect Avoid Variable Enhanced Response to Warfarin Decreased Response to Warfarin Diet Malnutrition Foods high in vitamin K such as: Beef liver Pork liver Green tea Leafy green vegetables Medications and nutritional supplements Quinidine Indomethacin Adrenal corticosteroids Anti-thyroid drugs Barbiturates Estrogen Aluminum hydroxide Medical conditions Reduced vitamin K absorption as occurs in: Obstructive jaundice Hepatitis Cirrhosis Diabetes mellitus Edema Hyperlipidemia Hypothyroidism Most errors in plasma-based coagulation testing occur in the pre-analytical phase.
10 A proper specimen is essential for accurate test results. The following table summarizes errors that can occur in specimen collection, transport, and storage. Preanalytical Factor Potential Error Recommendation Blood collection tube additive Some additives will interfere with coagulation test results. Use a collection tube containing sodium citrate. Order of draw Contamination from other additives could interfere with coagulation test results. Draw the coagulation tube (blue top) before tubes with other additives. Using a winged blood collection device for venipuncture Air that is collected into the blood specimen from the tubing dead space will alter the blood to anticoagulant Ratio and may impact test results. Use a discard tube to collect a sufficient amount of blood to fill the tubing dead space before collecting the patient's specimen. Collecting a coagulation specimen from a vascular access device (VAD) Heparin contamination occurs if the line had been previously flushed with heparin.