Transcription of NEW COVID - 19 CPT CODES - Maryland
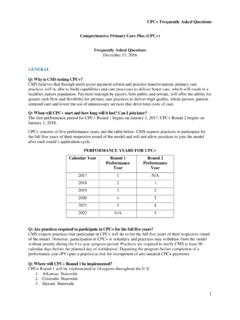
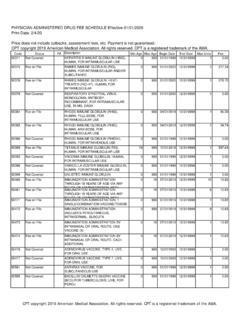
1 CPT CodeDefinition Effective DateImmunologyU0001 CDC 2019 Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel3/18/2020U00022019-nCoV Coronavirus, SARS-CoV-2/2019-nCoV ( COVID -19), any technique, multiple types or subtypes (includes all targets), non-CDC3/18/2020U0003 Infectious agent detection by nucleic acid (DNA or RNA); Severe Acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (Coronavirus disease [ COVID -19]), amplified probe technique, making use of high throughput technologies as described by CMS-2020-01-R 3/18/2020U00042019-nCoV Coronavirus, SARS-CoV-2/2019-nCoV ( COVID -19), any technique, multiple types or subtypes (includes all targets), non-CDC, making use of high throughput technologies as described by CMS-2020-01-R 3/18/202087635 Infectious agent detection by nucleic acid (DNA or RNA).
2 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [ COVID -19]), amplified probe technique3/13/202086328 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [ COVID -19])4/10/202086796 Antibody; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [ COVID -19])4/10/202087426 Infectious agent antigen detection by immunoassay technique, (eg, enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative, multiple-step method; severe acute respiratory syndrome coronavirus (eg, SARS-CoV, SARS-CoV-2 [ COVID -19])6/25/202086408 Neutralizing antibody, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [ COVID -19]).
3 Screen8/10/202086409 Titer8/10/202086413 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 9/8/202087635 Severe respiratory syndrome coronavirus 2 (SARSCoV-2) (Coronavirus disease [ COVID -19]), amplified probe technique10/6/202087636 Severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) (Coronavirus disease [ COVID -19]) and influenza virus types A and B, multiplex amplified probe technique10/6/202087637 Severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) (Coronavirus disease [ COVID -19]), influenza virus types A and B, and respiratorysyncytial virus , multiplex amplified probe technique10/6/2020 Supplies99072 Additional supplies, materials, and clinical staff time over and above those usually included in an office visit or other non-facility service(s), when performed during a Public Health Emergency as defined by law, due to respiratory -transmitted infectious disease9/8/2020 NEW COVID - 19 CPT CODES