Transcription of Principles of Metered-Dose Inhaler Design
1 Principles of Metered-Dose Inhaler DesignStephen P Newman PhDIntroduction: Development of the Pressurized Metered-Dose InhalerComponent Parts of the pMDIC ontainerPropellantsDrug FormulationMetering ValveActuatorDesigning pMDI productsAdvantages and Limitations of the pMDIB reath-Actuated pMDIsAutohalerEasibreatheK-HalerMD TurboXceloventSmartmistBreath-Coordinate d DevicesEasidoseBreath Coordinated InhalerOther Novel DevicesSpacehalerTempoBronchoAirSpacer DevicesSpacer DesignDrug Delivery From Spacer DevicesSummaryThe pressurized Metered-Dose Inhaler (pMDI) was introduced to deliver asthma medications in aconvenient and reliable multi- dose presentation.
2 The key components of the pMDI device (propel-lants, formulation, metering valve, and actuator) all play roles in the formation of the spray, andin determining drug delivery to the lungs. Hence the opportunity exists to Design a pMDI productby adjusting the formulation, metering-valve size, and actuator nozzle diameter in order to obtainthe required spray characteristics and fine-particle dose . Breath-actuated pMDIs, breath-coordi-nated pMDIs, spray-velocity modifiers, and spacer devices may be useful for patients who cannotuse a conventional press-and-breathe pMDI correctly. Modern pMDI devices, which contain non-ozone-depleting propellants, should allow inhalation therapy via pMDI to extend well into the 21stcentury for a variety of treatment words: Metered-Dose Inhaler , MDI, asthma, chronicobstructive pulmonary disease, spacer, aerosol, drug delivery.
3 [Respir Care 2005;50(9):1177 1188. 2005 Daedalus Enterprises]RESPIRATORYCARE SEPTEMBER2005 VOL50 NO91177 introduction : Development of the PressurizedMetered- dose InhalerIn the first half of the 20th century, inhaled drugs for thetreatment of asthma and chronic obstructive pulmonarydisease (COPD) were mostly delivered via hand-held,squeeze-bulb nebulizers. These devices were fragile, andsince the dose varied with hand pressure, they did notprovide consistent drug delivery. As described in detail byThiel,1 Riker Laboratories set out in the mid-1950s todevelop formulations of bronchodilator drugs in pressur-ized containers, providing greater convenience and a morereliable dose .
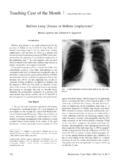
4 This development followed the introductionof proprietary cosmetic aerosols as pressure-packs, andcoincided with the invention of a metering valve capableof providing the patient with at least 100 precise pressurized Metered-Dose Inhaler (pMDI, Fig. 1)quickly became the most important device for deliveringinhaled drugs, and today approximately 500 million areproduced , they were given the acronym MDI, but the term pMDI is preferable, in order todistinguish them from dry powder inhalers (DPIs) andother nonpressurized devices, some of which also have amulti- dose capability.
5 The pMDI was once termed themost complex dosage form used in medicine today, 3al-though with the development of increasingly sophisticatedDPIs and microprocessor-controlled nebulizers, this mayno longer be Parts of the pMDIThe pMDI comprises several components (Fig. 2), eachof which is important to the success of the whole components are container, propellants, drug formu-lation, metering valve, and actuator. It was demonstratedmany years ago that the aerosol size from a pMDI could beinfluenced by a variety of factors associated with thesecomponents,4as listed in Table pMDI container must be able to withstand the highpressure generated by the propellant, it must be made ofinert materials, and it must be sufficiently robust.
6 The firstprototype pMDI was formulated in a CocaCola bottle,1and smaller (10 mL) glass bottles were used in the earliestmarketed pMDIs. Stainless steel has also been used as apMDI container material. Aluminum is now preferred,since, compared to glass, it is lighter, more compact, lessfragile, and light-proof. Coatings on the internal containersurfaces may be useful to prevent adhesion of drug parti-cles and chemical degradation of P Newman PhD is affiliated with Pharmaceutical Profiles Ltd,Nottingham, United P Newman PhD presented a version of this article at the 36thRESPIRATORYCAREJ ournal Conference, Metered-Dose Inhalers and DryPowder Inhalers in Aerosol Therapy, held April 29 through May 1, 2005,in Los Cabos, Profiles Ltd has received research grants directly relatedto the subject matter of this article from the following corporations.
7 Altana Pharma, Aventis, Forest Laboratories, Fujisawa, Ivax, and : Stephen P Newman PhD. E-mail: 1. Schematic of typical pressurized Metered-Dose 2. The key component parts of the pressurized OFMETERED-DOSEINHALERDESIGN1178 RESPIRATORYCARE SEPTEMBER2005 VOL50 NO9 PropellantsPropellants in pMDIs are liquefied compressed gasesthat are in the gaseous phase at atmospheric pressure, butform liquids when compressed. They are required to benontoxic, nonflammable, compatible with drugs formu-lated either as suspensions or solutions, and to have ap-propriate boiling points and ensure consis-tent dosing, the vapor pressure must be constant throughoutthe product s life, and this rules out the use of compressedgases such as carbon dioxide, the pressure of which woulddecrease as doses were (CFCs) meet the required criteria,and pMDIs have traditionally been formulated with thehighly volatile CFC-12 (dichlorodifluoromethane) as themajor component.
8 CFC-11 (trichlorofluoromethane) orCFC-114 (dichlorotetrafluoroethane), which have higherboiling points, may be used to modify the vapor pressure,and to facilitate preparation of the formulation. CFC-12may be added to the container either at low temperature,before the metering valve is crimped in place (cold-fill-ing), or via the metering valve after crimping (pressure-filling). A key property of CFCs is that within a closedcontainer they form a 2-phase (liquid and saturated vapor)system, such that a dynamic equilibrium exists betweenliquid and vapor phases, giving a constant vapor pressureirrespective of whether the can is full or nearly empty.
9 Thevapor pressure inside a pMDI is typically 300 500 kPa(3 5 atmospheres or 2,250 3,750 mm Hg), depending onthe propellant mixture, the presence of other excipients(surfactants and other inactive components of the formu-lation), and ambient , the use of CFCs was banned under interna-tional agreement, because the release of chlorine duringtheir degradation damages the ozone layer in the containing one of 2 hydrofluoroal-kanes (tetrafluoroethane [HFA-134a] and heptafluoropro-pane [HFA-227]) are now appearing on the of pMDIs with HFA propellants has led tomany challenges, often involving the development of newexcipients and metering ,10 HFA-134a and HFA-227 have broadly similar thermodynamic properties toCFC-12, but no HFA equivalent to CFC-11 or CFC-114 isavailable, so excipients with lower volatility may be re-quired to modify the vapor pressure.
10 This issue also influ-ences the filling methods that are feasible for HFA prod-ucts, and has led to the development of novel pressure-filling has also been necessary to undertakeextensive toxicity testing on the new propellants, and thento conduct clinical trials demonstrating safety and efficacyof new drug formulations. Taking into account the timerequired for reformulation, toxicity testing, and clinicaltesting, it has been estimated that development of a newHFA formulation of an inhaled drug takes about 10 CFC replacement continues, an essential-use ex-emption was granted for pulmonary inhalers, allowing thecontinued use of CFCs in recognition of the unique healthbenefits that pMDIs are greenhouse gases, 6which could lead tofuture restrictions on their use, although their contributionto global warming is likely to be very , propane, or butane could be considered as propel-lants.