Transcription of Reference ID: 3115694
1 0873 333-33-100411 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ADIPEX-P safely and effectively.
2 See full prescribing information for ADIPEX-P . ADIPEX-P (phentermine hydrochloride USP) CIV for oral use Initial Approval: 1959 ---- INDICATIONS AND USAGE --- ADIPEX-P is a sympathomimetic amine anorectic indicated as a short-term adjunct (a few weeks) in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity for patients with an initial body mass index 30 kg/m2, or 27 kg/m2 in the presence of other risk factors ( , controlled hypertension, diabetes, hyperlipidemia). (1) The limited usefulness of agents of this class, including ADIPEX-P , should be measured against possible risk factors inherent in their use. (1) - DOSAGE AND ADMINISTRATION Dosage should be individualized to obtain an adequate response Rare cases of serious regurgitant cardiac valvular disease have been reported.
3 ( ) Tolerance to the anorectic effect usually develops within a few weeks. If this occurs, phentermine should be discontinued. The recommended dose should not be exceeded. ( ) Phentermine may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle. ( ) Risk of abuse and dependence. The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage. ( ) Concomitant alcohol use may result in an adverse drug reaction. ( ) Use caution in patients with even mild hypertension (risk of increase in blood pressure). ( ) A reduction in dose of insulin or oral hypoglycemic medication may be required in some patients.
4 ( ) ------ ADVERSE REACTIONS ----- 10 OVERDOSAGE 14 CLINICAL STUDIES Acute Overdosage 16 HOW SUPPLIED/STORAGE Chronic Intoxication AND HANDLING 11 DESCRIPTION 17 PATIENT COUNSELING 12 CLINICAL PHARMACOLOGY INFORMATION Mechanism of Action *Sections or subsections omitted Pharmacodynamics from the full prescribing information Pharmacokinetics are not listed. 13 NONCLINICAL TOXICOLOGY Carcinogenesis, Mutagenesis, Impairment of Fertility FULL PRESCRIBING INFORMATION 1 INDICATIONS AND USAGE ADIPEX-P is indicated as a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity for patients with an initial body mass index 30 kg/m2, or 27 kg/m2 in the presence of other risk factors ( , controlled hypertension, diabetes, hyperlipidemia).
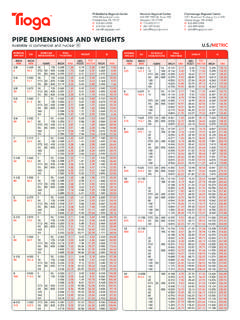
5 Below is a chart of body mass index (BMI) based on various heights and weights. BMI is calculated by taking the patient s weight, in kilograms (kg), divided by the patient s height, in meters (m), squared. Metric conversions are as follows: pounds = kg; inches x = meters. BODY MASS INDEX (BMI), kg/m2 Height (feet, inches) Weight 5'0" 5'3" 5'6" 5'9" 6'0" 6'3"(pounds) Primary Pulmonary Hypertension Primary Pulmonary Hypertension (PPH) a rare, frequently fatal disease of the lungs has been reported to occur in patients receiving a combination of phentermine with fenfluramine or dexfenfluramine. The possibility of an association between PPH and the use of phentermine alone cannot be ruled out; there have been rare cases of PPH in patients who reportedly have taken phentermine alone.
6 The initial symptom of PPH is usually dyspnea. Other initial symptoms may include angina pectoris, syncope or lower extremity edema. Patients should be advised to report immediately any deterioration in exercise tolerance. Treatment should be discontinued in patients who develop new, unexplained symptoms of dyspnea, angina pectoris, syncope or lower extremity edema, and patients should be evaluated for the possible presence of pulmonary hypertension. Valvular Heart Disease Serious regurgitant cardiac valvular disease, primarily affecting the mitral, aortic and/or tricuspid valves, has been reported in otherwise healthy persons who had taken a combination of phentermine with fenfluramine or dexfenfluramine for weight loss. The possible role of phentermine in the etiology of these valvulopathies has not been established and their course in individuals after the drugs are stopped is not known.
7 The possibility of an association between valvular heart disease and the use of phentermine alone cannot be ruled out; there have been rare cases of valvular heart disease in patients who reportedly have taken phentermine alone. Development of Tolerance, Discontinuation in Case of Tolerance When tolerance to the anorectant effect develops, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued. Effect on the Ability to Engage in Potentially Hazardous Tasks Adverse events have been reported 140 27 25 23 21 19 18 29 27 24 22 20 19 31 28 26 24 22 20 33 30 28 25 23 21 35 32 29 27 25 23 37 34 31 28 26 24 39 36 32 30 27 25 41 37 34 31 29 26 43 39 36 33 30 28 45 41 37 34 31 29 with the lowest effective dose. (2) R2 3 7 3 5 Late evening administration should be avoided (risk of Phentermine may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; the patient should therefore be cautioned accordingly.)
8 Risk of Abuse and Dependence Phentermine is related chemically and pharmacologically to amphetamine (d- and dll-amphetamine) and other related stimulant drugs have been extensively abused. The possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. See Drug Abuse and Dependence (9) and Overdosage (10). in the cardiovascular, central 150 nervous, gastrointestinal, allergic, 160 and endocrine systems. (6) 170insomnia). (2) ADIPEX-P can be taken with To report SUSPECTED ADVERSE 180 REACTIONS, contact TEVA USA, or without food. ( ) 190 PHARMACOVIGILANCE at- DOSAGE FORMS AND STRENGTHS 2001-888-838-2872, X6351 orRev. T 1/2012 ADIPEX-P (PhentermineHydrochloride USP, mg) 009 only 019 Capsules containing mg or 210phentermine hydrochloride.
9 (3) FDA at 1-800-FDA-1088 or 220 Tablets containing mg 230phentermine hydrochloride. (3) The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of DRUG INTERACTIONS ----- 240 474339 36 3330------ CONTRAINDICATIONS ----- History of cardiovascular Monoamine oxidase inhibitors: 250494440 37 3431 Risk of hypertensive crisis. (4, ) Usage With Alcohol disease ( , coronary artery disease, stroke, arrhythmias, Alcohol: Consider potential The limited usefulness of agents of this class, including ADIPEX-P , Concomitant use of alcohol with phentermine may result in an adverse interaction ( ) drug reaction.[see Clinical Pharmacology ( , )] should be measured against congestive heart failure, uncontrolled hypertension) (4) Insulin and oral hypoglycemics: Requirements may be altered.
10 Possible risk factors inherent in their use such as those described below. Use in Patients With Hypertension 2 DOSAGE AND ADMINISTRATION Use caution in prescribing phentermine for patients with even mild During or within 14 days following ( ) Exogenous Obesity hypertension (risk of increase in blood pressure).the administration of monoamine oxidase inhibitors (4) Adrenergic neuron blocking Dosage should be individualized to obtain an adequate response with Use in Patients on Insulin or Oral Hypoglycemicdrugs: Hypotensive effect may the lowest effective dose. Hyperthyroidism (4) Medications for Diabetes Mellitusbe decreased by phentermine. Glaucoma (4) Agitated states (4) The usual adult dose is one capsule ( mg) daily as prescribed A reduction in insulin or oral hypoglycemic medications in patients with( ) diabetes mellitus may be the physician, administered before breakfast or 1 to 2 hours after- USE IN SPECIFIC POPULATIONS History of drug abuse (4) Pregnancy (4, ) Nursing (4, ) breakfast for appetite control.