Transcription of THE UNIVERSITY OF THE STATE OF NEW YORK • …
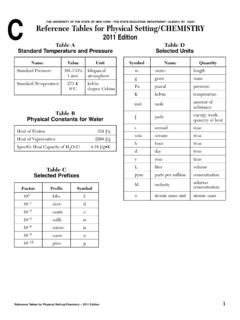
1 Reference Tables for Physical Setting/ CHEMISTRY1 THE UNIVERSITY OF THE STATE OF NEW YORK THE STATE EDUCATION DEPARTMENT ALBANY, NY 12234 Reference Tables for Physical Setting/ CHEMISTRY2002 EditionTable AStandard Temperature and PressureTable BPhysical Constants for WaterTable CSelected PrefixesTable ESelected Polyatomic IonsTable DSelected UnitsNameValueUnitStandard kPa kilopascal1 atm atmosphereStandard Temperature273 K kelvin0 Cdegree CelsiusHeat of Fusion334 J/gHeat of Vaporization2260 J/gSpecific Heat Capacity of H2O ( )
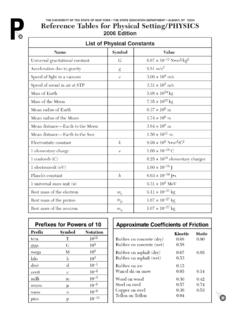
2 J/g CFactorPrefixSymbol103kilo-k10 1deci-d10 2centi-c10 3milli-m10 6micro- 10 9nano-n10 12pico-pSymbolNameQuantitymmeterlengthgg rammassPapascalpressureKkelvintemperatur emolmoleamountof substanceJjouleenergy, work,quantity of heatssecondtimeLlitervolumeppmpart per millionconcentrationMmolaritysolutioncon centrationH3O+hydroniumHg22+dimercury (I)NH4+ammoniumC2H3O2 acetateCH3 COO }CN cyanideCO32 carbonateHCO3 hydrogen carbonateC2O42 oxalateClO hypochloriteClO2 chloriteClO3 chlorateClO4 perchlorateCrO42 chromateCr2O72 dichromateMnO4 permanganateNO2 nitriteNO3 nitrateO22 peroxideOH hydroxidePO43 phosphateSCN thiocyanateSO32 sulfiteSO42 sulfateHSO4 hydrogen sulfateS2O32 thiosulfateC2 Reference Tables for Physical Setting/ CHEMISTRYT able FSolubility Guidelines for Aqueous Solutions0 102030405060708090100 Temperature ( C)Solute per 100 g of H2O (g)
3 1401301201101009080706050403020100 KINaNO3NH3 HClNaClKNO3NH4 ClKClSO2 KClO3 table GSolubility CurvesIons That FormSolubleCompoundsExceptionsGroup 1 ions (Li+, Na+, etc.)ammonium (NH4+)nitrate (NO3 )acetate (C2H3O2 or CH3 COO ) hydrogen carbonate(HCO3 )chlorate (ClO3 )perchlorate (ClO4 )halides (Cl , Br , I )when combined with Ag+, Pb2+, and Hg22+sulfates (SO42 )when combined with Ag+, Ca2+, Sr2+, Ba2+, and Pb2+Ions That FormInsolubleCompoundsExceptionscarbonat e (CO32 )when combined with Group 1ions or ammonium (NH4+)chromate (CrO42 )when combined with Group 1ions, Ca2+, Mg2+, or ammonium (NH4+)phosphate (PO43 )when combined with Group 1ions or ammonium (NH4+)sulfide (S2 )
4 When combined with Group 1ions or ammonium (NH4+)hydroxide (OH )when combined with Group 1ions, Ca2+, Ba2+, Sr2+, orammonium (NH4+)Reference Tables for Physical Setting/ CHEMISTRY3 table HVapor Pressure of Four Liquids0255075100125200150100500 Vapor Pressure (kPa)Temperature ( C) kPapropanoneethanolwaterethanoicacid4 Reference Tables for Physical Setting/ CHEMISTRYT able IHeats of Reaction at kPa and 298 KTable JActivity Series**Reaction H(kJ)*CH4(g) + 2O2(g) CO2(g) + 2H2O( ) (g) + 5O2(g) 3CO2(g) + 4H2O( ) ( ) + 25O2(g) 16CO2(g) + 18H2O( ) 109432CH3OH( ) + 3O2(g) 2CO2(g) + 4H2O( ) 1452C2H5OH( ) + 3O2(g) 2CO2(g) + 3H2O( ) 1367C6H12O6(s) + 6O2(g) 6CO2(g) + 6H2O( ) 28042CO(g) + O2(g) 2CO2(g) (s) + O2(g) CO2(g) (s) + 3O2(g) 2Al2O3(s) 3351N2(g) + O2(g) 2NO(g)+ (g) + 2O2(g) 2NO2(g)+ (g) + O2(g) 2H2O(g) (g) + O2(g) 2H2O( ) (g) + 3H2(g) 2NH3(g)
5 (s) + 3H2(g) C2H6(g) (s) + 2H2(g) C2H4(g)+ (s) + H2(g) C2H2(g)+ (g) + I2(g) 2HI(g)+ (s) H2OK+(aq) + NO3 (aq)+ (s) H2 ONa+(aq) + OH (aq) (s) H2 ONH4+(aq) + Cl (aq)+ (s) H2 ONH4+(aq) + NO3 (aq)+ (s) H2 ONa+(aq) + Cl (aq)+ (s) H2 OLi+(aq) + Br (aq) +(aq) + OH (aq) H2O( ) *Minus sign indicates an exothermic **H2 CuAgAuF2Cl2Br2I2**Activity Series based on hydrogen standardNote:H2is nota metalReference Tables for Physical Setting/ CHEMISTRY5 table KCommon AcidsTable LCommon BasesTable NSelected RadioisotopesTable MCommon Acid Base IndicatorsFormulaNameHCl(aq)hydrochloric acidHNO3(aq)nitric acidH2SO4(aq)sulfuric acidH3PO4(aq)phosphoric acidH2CO3(aq) orcarbonic acidCO2(aq)CH3 COOH(aq) ethanoic acid or HC2H3O2(aq)(acetic acid)FormulaNameNaOH(aq)sodium hydroxideKOH(aq)potassium hydroxideCa(OH)2(aq)calcium hydroxideNH3(aq)
6 Aqueous ammoniaApproximate IndicatorpH Range Colorfor ColorChangeChangemethyl to yellowbromthymol to 10colorless to to bluebromcresol to bluethymol to blueNuclideHalf-LifeDecay Nuclide d gold-19814C5730 y carbon-1437Ca175 ms + y y min + s y d s + h y s s + d 104y plutonium-239226Ra1600 y d y 105y 1010y 105y 108y 109y uranium-238ms = milliseconds; s = seconds; min = minutes;h = hours; d = days; y = yearsNameGeneralExamplesFormulaNameStruc tural Formulaalkanes CnH2n+2ethanealkenesCnH2nethenealkynesCn H2n 2ethyne6 Reference Tables for Physical Setting/ CHEMISTRYT able OSymbols Used in Nuclear ChemistryTable POrganic PrefixesTable QHomologous Series of HydrocarbonsNameNotationSymbolalpha particle42He or 42 beta particle (electron)
7 0 1e or 0 1 gamma radiation00 neutron10nnproton11H or 11pppositron0+1e or 0+1 +PrefixNumber of Carbon Atomsmeth-1eth-2prop-3but-4pent-5hex-6he pt-7oct-8non-9dec-10 HHHHHHCCCCHHHCCHHHn= number of carbon atomsORCHORCOR OCH3 CCH2CH2CH32-pentanoneORCR OCHR eference Tables for Physical Setting/ CHEMISTRY7 table ROrganic Functional GroupsClass ofCompoundFunctionalGroupGeneralFormulaE xamplehalide(halocarbon)alcoholetheralde hydeF (fluoro-)Cl (chloro-)Br (bromo-)I (iodo-)OHRX(Xrepresentsany halogen)ROHROR CH3 CHClCH32-chloropropaneCH3CH2CH2OH1-propa nolCH3 OCH2CH3methyl ethyl etheramideOOCOHOCOOCNHNOCOCH3CH2 CHpropanalOR RCNHR RNR ORCOH ketoneorganic acidesteramineOCH3CH2 COHpropanoic acidOCH3CH2 COCH3methyl propanoateCH3CH2CH2NH21-propanamineOCH3C H2 CNH2propanamideRrepresents a bonded atom or groupof atoms.