Transcription of TOLERABLE UPPER INTAKE LEVELS FOR VITAMINS AND …
1 Scientific Committee on Food Scientific Panel on Dietetic Products, Nutrition and AllergiesTOLERABLE UPPER INTAKE LEVELS FOR VITAMINS AND MINERALS European Food Safety AuthorityFebruary 2006 European Food Safety Authority 2006 ISBN: 92-9199-014-0 Reproduction is authorized, provided the source is acknowledged, save where otherwise views or positions expressed in this report do not represent in legal terms the official position neither of the European Food Safety Authority or the European Commission. The European Food Safety Authority assumes no responsibility or liability for any errors or inaccuracies that may appear. About EFSAF ollowing a series of food scares in the 1990s ( BSE, dioxins) which undermined consumer confidence in the safety of the food chain, the European Union (EU) concluded that it needed to establish a new scientific body charged with providing independent advice on food safety issues associated with the food chain.
2 Its primary objective was to contribute to a high level of consumer health protection in the area of food safety. To voice concerns about food safety and the ability of regulatory authorities to fully protect consumers, the result was the establishment of the European Food Safety Authority (EFSA) was established funded by the European Community as an independent agency in close collaboration with national authorities and in open consultation with its stakeholders, EFSA provides independent scientific advice on all matters linked to food and feed safety - including animal health and welfare and plant protection - and provides scientific advice on nutrition in relation to Community s work falls into two areas: risk assessment and risk communication.
3 In particular, EFSA s risk assessment provides risk managers (EU institutions with political accountability, the European Commission, European Parliament and Council) with a sound scientific basis for defining policy-driven legislative or regulatory measures required to ensure a high level of consumer protection with regards to food and feed communicates to the public in an open and transparent way on all matters within its and analysis of scientific data, identification of emerging risks and scientific support to the Commission, particularly in case of a food crisis, are also part of EFSA s mandate, as laid down in the founding Regulation (EC) No 178/2002 of 28 January more information about the European Food Safety Authority, visit the EFSA home page at: Table of ContentsForeword.
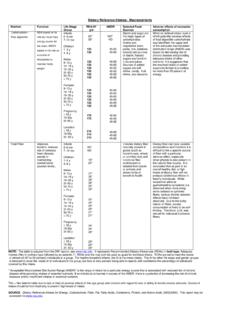
4 3 History ..5 About the SCF ..5 About the NDA Panel ..5 Background ..7 Terms of Reference ..7 Contributors and Special Acknowledgments ..8 Scientific Opinions :Guidelines ..9 Beta carotene ..15 Vitamin B6 ..29 Vitamin B12 ..45 Folate ..51 Manganese ..59 Selenium ..65 Molybdenum ..77 Vitamin B2 ..87 Vitamin B1 ..93 Biotin ..99 Magnesium ..107 Pantothenic acid ..117 Nicotinic acid and nicotinamide (niacin) ..121 Iodine ..135 Preformed vitamin A (retinol and retinyl esters) ..151 Vitamin D ..167 Zinc ..191 Copper ..203 Calcium ..215 Vitamin E ..243 Vitamin K ..253 Chromium ..261 Vanadium ..273 Silicon ..287 Vitamin C (L-ascorbic acid, its calcium, potassium and sodium salts and L-ascorbyl-6-palmitate).
5 295 Boron (sodium borate and boric acid) ..309 Iron ..325 Nickel ..347 Fluoride ..363 Potassium ..409 Chloride ..423 Sodium ..429 Phosphorus ..447 Tin ..461 1 2 3 scientific opinions presented in this compilation were developed at the request of the European Commission by the Scientific Committee on Food (SCF) (up to April 2003) and the Scientific Panel on Dietetic Products, Nutrition and Allergies (NDA) of EFSA (May 2003 to 2005). The context of this request was the need for scientific advice on the safety of VITAMINS and minerals to support the implementation of impending harmonized EU legislation for food supplements and fortified foods, and particularly to assist with the setting of maximum limits for micronutrients in these opinions present comprehensive evaluations of possible adverse health effects of individual micronutrients at intakes in excess of dietary requirements and, where possible, establish TOLERABLE UPPER INTAKE LEVELS (UL) for different population groups.
6 The approach taken was based on the principles of scientific risk assessment. Both the SCF and the NDA Panel, in turn, established a working group to prepare draft opinions which were then considered, revised and adopted at plenary meetings of the SCF or the NDA Panel. This working group, which I was privileged to chair, comprised of experts in toxicology and nutrition drawn from the SCF or NDA Panel, complemented by additional experts. Sincere appreciation is due to the many scientists whose dedication and commitment made the completion of these reports possible. In particular, I would like to thank those who prepared draft reports - Jan Alexander, Wulf Becker, Maxine Bonham, Veronique Aza s Braesco, Angelo Carere, Hans Classen, Peter Elias, Ibrahim Elmadfa, Werner Grunow, Alan Jackson, Andreu Palou, Hildegard Przyrembel, Andy Renwick, Wim Saris, Eberhard Schmidt, Klaus Sch mann, Gerrit Speijers, Sean Strain, Henk van den Berg and Ron Walker.
7 I also thank the EFSA and EC staff who supported this work, in particular Pilar Rodr guez Iglesias and Leng Heng (Secretariat of the NDA Panel), Miguel ngel Granero Rosell, (Secretariat of the SCF), and Helen Lee, Basil Mathioudakis and Sabine Osaer (DG Health and Consumer Protection).These opinions will find immediate application by the regulatory agencies that oversee addition of micronutrients to foods and food supplements in the EU. They also represent a valuable scientific reference on the safety of micronutrients which will be used by scientists and policy makers for many years. Albert Flynn, ChairEFSA Scientific Panel on Dietetic Products, Nutrition and Allergies December, 2005 Foreword 4 History ABOUT THE SCFThe Scientific Committee for Food (SCF)
8 Was originally established by Commission Decision 74/234/EEC of 16 April 1974, replaced by Commission Decision 95/273/EC of 6 July 1995, to advise the European Commission on matters relating to the protection of the health and safety of persons arising or likely to arise from the consumption of food, in particular on nutritional, hygienic and toxicological the reorganisation of the Commission s Scientific Committees in 1997, the previous Decisions were replaced by Commission Decision 97/579/EC of 23 July 1997 setting up eight Scientific Committees, including the Scientific Committee on Food (SCF).The members of the SCF were independent persons, highly qualified in the fields associated with medicine, nutrition, toxicology, biology, chemistry, or other related Secretariat of the Scientific Committees was provided by the services of the Commission.
9 Most of Community Directives and Regulations related to foodstuffs, required the Commission to consult the Committee on provisions which may have an effect on public health falling within the scope of these directives and SCF mandate consisted of advising the European Commission on scientific and technical questions concerning consumer health and food safety associated with the consumption of food products and in particular questions relating to toxicology and hygiene in the entire food production chain, nutrition, and applications of agrifood technologies, as well as those relating to materials coming into contact with foodstuffs, such as THE NDA PANELWith the establishment of EFSA by the European Parliament and Council Regulation (EC) No 178/2002, the scientific advice provided by the previous Scientific Committees was handed over to EFSA.
10 In accordance with its founding Regulation, EFSA is required to provide scientific advice and scientific and technical support for the Community s legislation and policies in all fields which have a direct or indirect impact on food and feed safety. It is required to provide independent information on all matters within these fields and communicate on risks. The Scientific Committee and Scientific Panels of EFSA are responsible for providing the scientific opinions of the Authority, each within their own spheres of competence. EFSA is required to contribute to a high level of protection of human life and health, and in this respect take account of animal health and welfare, plant health and the environment, in the context of the operation of the internal Article 18 of the Decision concerning the establishment and operations of the Scientific Committee and Panels, adopted by the Authority s Management Board on 17 October 2002, the Scientific Panel on Dietetic Products, Nutrition and Allergies (NDA Panel) deals with questions relating to dietetic products, human nutrition and food allergy, and other associated subjects such as novel foods.