3 Electron Paramagnetic Resonance
Found 9 free book(s)Experiment 7 Electron Spin Resonance (ESR)
physics.unm.eduUsing ESR (Electron Spin Resonance, also known as Electron Paramagnetic Resonance) you will be measuring one of the best known quantities in all of physics, the famous g s-factor of the electron. This will be achieved by looking for the “spin-flip” transition of a free (unpaired) electron exposed to a magnetic field. 7.1.1 ESR in Theory
Lec 6 - Phosphine & Carbene Ligands
alpha.chem.umb.eduof electron density from the metal centre. 6 ... –not resonance forms (sinlget↔ triplet resonance forbidden) ... e.g. just as paramagnetic triplet :CH 2can dimerizeto form diamagnetic H 2C=CH 2, it also binds to a triplet LnMfragment to give a diamagnetic LnM=CH 2complex.
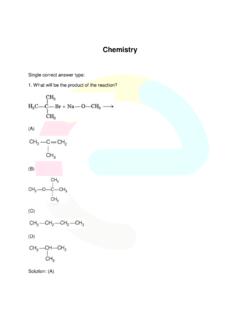
Chemistry
d2cyt36b7wnvt9.cloudfront.netand are isoelectronic and contains one unpaired electron each. Thus, both are paramagnetic. III. In carbonate ion. all three bonds are identical due to resonance IV. is more stable than due to inert pair effect, hence, prefers to form divalent compounds. Thus, the incorrect statements are III …
Module 2 Spectroscopic techniques Lecture 3 Basics of ...
www.nptel.ac.inelectron paramagnetic resonance spectroscopy. iii. Infrared radiation: The energies associated with molecular vibrations fall in the infrared region of electromagnetic spectrum. Infrared spectroscopy is therefore also known as vibrational spectroscopy and is a very useful
Chapter 6 Molecular Fluorescence and Phosphorescence
www.philadelphia.edu.joPhosphorescence involves change in electron spin and may endure for several seconds. In most cases, photoluminescent radiation tends to be at ... • Resonance radiation ... Molecule is paramagnetic in the T excited state and diamagnetic in the S excited state 2. S T transitions (or reverse) are less probable
ELECTRON SPIN RESONANCE (ESR) SPECTROSCOPY
www.nou.ac.inElectron spin resonance (ESR), also called electron paramagnetic resonance (EPR), is a spectroscopic technique confined to the study of species having one or more unpaired electrons. Among the large number of systems having one or more unpaired electrons, i.e. paramagnetic system, the most important ones are free radicals, transition metal ions ...
Electron Paramagnetic Resonance Theory E. Duin
webhome.auburn.eduElectron Paramagnetic Resonance Theory E. Duin . 1 - 2 1. Basic EPR Theory 1.1 Introduction This course manual will provide the reader with a basic understanding needed to be able to get useful information using the technique of electron …
NMR Spectroscopy
casegroup.rutgers.eduN.M.R. = Nuclear Magnetic Resonance Basic Principles ... 0 = 9.5 T) is 3.8 x 10-5 Kcal/mol N /N =1.000064 The surplus population is small (especially when compared to UV or IR). ... more precisely on the local electron distribution this effect is called the chemical shift. NMR Spectroscopy The Chemical Shift Nuclei, however, in molecules are ...
Lecture Notes on Superconductivity (A Work in Progress)
courses.physics.ucsd.eduLecture Notes on Superconductivity (A Work in Progress) Daniel Arovas Congjun Wu Department of Physics University of California, San Diego June 23, 2019