Search results with tag "Electron"
Science with Ms. McLean - Home
mcleanscience.weebly.comelectrons 4. (3 num r of valence electr ns 5. number of electron(s) it has to lose to become stable 6. number of shells holding the maximum number of electrons 19 G. 20 Circle the letter of the best answer. 7. What is the maximum number of electrons that the first electron shell can hold? 2007 McGraw-Hill Ryerson Limited Section 2.3 37
Levels, Sublevels, Orbitals, and Electrons!!!
web.gccaz.eduThe sublevels contain orbitals. Orbitals are spaces that have a high probability of containing an electron. In other words, an orbital is an area where the electrons live. There can be two electrons in one orbital maximum. The s sublevel has just one orbital, so can contain 2 electrons max. The p sublevel has 3 orbitals, so can contain 6 ...
Average Mark: 20.9 /30 42.2 /70 19.2 /30 - JABchem
jabchem.org.ukA Hund’s Rule: Electron half -fill degenerate orbitals before doubly filling orbitals B Aufbau Principle: Electrons fill in order of increasing energy C Pauli Exclusion Principle: Orbital can hold 2 electrons only and they have opposite spins D Valence Shell Electron Pair Repulsion Theory: predicts geometry of molecules 3 C 94
分子結晶の電子構造(バンド構造) - Kyoto U
kuchem.kyoto-u.ac.jpElectron clouds have to be extended outer side to increase the intermolecular MO overlap lone-pair electrons π-electrons In most cases, MO overlap is not extended to the whole crystal due to localized character of the electron cloud For the π-conjugated systems, MO overlap can be extended to the whole crystal
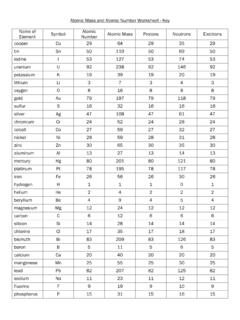
Atomic Protons Neutrons Electrons Lewis Dot Mass
www.lcps.orgUse the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li Magnesium 12 24 12 12 12 Mg:
Bohr Model Worksheet - Duplin County Schools
www.duplinschools.netFor each element draw the inner electrons blue & the valence (outer) electrons red. The circles represent possible electron shells. ... Element Symbol Protons Neutrons Electrons Lithium Li 3 7-3=4 3 carbon C 6 12-6=6 6 Sodium Aluminum Pb Ti Zn 80 17 Tungsten . Title: Bohr Model Worksheet Author: varuiz Created Date ...
1. Semiconductor Materials & Physics
www.cityu.edu.hk1.6 Electron Mobility Using the theorem of equipartition of energy, m n v th 2/2 = 3kT/2, where m n is the electron effective mass and v th is the average thermal velocity. Electrons in the semiconductor therefore move rapidly in all directions. The thermal motion of an individual electron can be visualized as a succession of random scattering from
GCE Chemistry A
www.ocr.org.ukAND highest energy or outer electron is in a s orbital or s sub–shell 1 1.1 ALLOW ‘outer’ or ‘valence’ for ‘highest energy’ IGNORE. electron configurations . DO NOT ALLOW. ... electron repulsion between shells . IGNORE. just ‘shielding’ ALLOW. more/stronger/bigger nuclear
Homework Chapter 28: Magnetic Fields
www4.uwsp.eduassume that the electron’s velocity vector is perpendicular to the electric field vector between the plates. In unit-vector notation, what uniform magnetic field allows the electron to travel in a straight line in the gap? 28.23 What uniform magnetic field, applied perpendicular to a beam of electrons moving at 1.30 × 106 m/s, is required to
Lewis Dot Structures and Molecule Geometries Worksheet ...
www.teachengineering.org1. Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. You can quickly refer to the periodic table for the group A number for this information. In the case of polyatomic anions, add the electrons represented by the negative charge to the total number of valence electrons.
Experiment 4: Charge to mass ratio (e/m) of the electron
www.columbia.eduPHYS 1493/1494/2699: Exp. 4 – e/m of the electron 2 Introduction Our first measurement of atomic structure Charge-to-mass ratio of electron: Motivation and history of the first e/m measurement Consequences Thomson’s experiment The physics behind the experiment: The magnetic field generated by a single loop Charged particle in constant magnetic field
CHM151LL: VSEPR and Molecular Geometry Tables
web.gccaz.eduCHM151LL: VSEPR and Molecular Geometry Tables Valence-Shell Electron-Pair Repulsion (VSEPR) model Lewis structures show the two-dimensional distribution of atoms and electrons. The molecular geometry, or three-dimensional shape of a molecule or polyatomic ion, can be determined using valence-shell electron-pair repulsion (abbreviated VSEPR and ...
NEET 2021 Question paper & Solutions PDF by Embibe …
d2cyt36b7wnvt9.cloudfront.net1. An infinitely long straight conductor carries a current of 5 as shown. An electron is moving with a speed of 105m/s parallel to the conductor. The perpendicular distance between the electron and the conductor is 20cm at an instant. Calculate the magnitude of the force experienced by the electron at that instant. (1) 4×10−20N (2) 8 ×10−20N
The 16 and 18 Electron Rule in Organometallic Chemistry ...
www.yorku.cacalled the 16 and 18 Electron Rule. Careful subsequent study has almostinvari- ably shown that the original formulation was incorrect. The accessibility of 16 and 18 electron configurations also has important consequences for mechanisms of organometallic reactions, as outlined below.
24.01 - vtu.ac.in
vtu.ac.inPrinciple, construction and working of X-ray Diffractometer, crystal size determination by Scherrer equation. Principle, construction, working and applications of -Atomic Force Microscope (AFM), X-ray Photoelectron Spectroscope (XPS), Scanning Electron Microscope (SEM), Transmission Electron Microscope (TEM) Numerical problems.
Worksheet 13 - Molecular Shapes Lewis structures by using ...
butane.chem.uiuc.eduWorksheet 13 - Molecular Shapes The shapes of molecules can be predicted from their Lewis structures by using the VSEPR (Valence Shell Electron Pair Repulsion) model, which states that electron pairs around a central atoms will assume a geometry that keeps them as far apart from each other as possible. This is illustrated by the drawings below.
Chapter 2 Semiconductor Heterostructures - Cornell …
courses.cit.cornell.eduThe valence band offset is then, ... As more electrons and holes diffuse, the strength of the electric field increases until the drift components of the electron and hole currents are exactly equal and opposite to their respective diffusive components. When this has happened the junction is in equilibrium.
Physics Notes Class 12 Chapter 14 Semiconductor ...
ncerthelp.comare the number density of electrons and holes and n i is number density of intrinsic carriers, i.e., electrons or holes. In n-type semiconductor, n e > > n h In p -type semiconductor, n h > > n e Electrical conductivity of extrinsic semiconductor is given by σ = 1 / ρ = e (n e μ e + n h μ h) where ρ is resistivity, μ e and μ h
Writing & Naming Formulas of Ionic ... - Mrs. Jill Perkins
scienceperks.weebly.comThe valence of an elements is the charge an atom takes when it loses or gains electrons and becomes an ion. Metal atoms lose 1, 2 or 3 electrons and become + ions (cations)
Atomic Neutrons Electrons Atomic Charge Protons mass
www.everettcc.eduCr 24 24 28 21 52 +3 F 9 9 10 9 19 0 Nb 41 41 52 35 93 +5 P 15 15 16 18 31 -3 Rb 37 37 48 36 85 +1 Pd 46 46 60 46 106 0 Os 76 76 114 72 190 +4 K 19 19 20 19 39 0 Mo 42 42 54 36 96 +6 Sg 106 106 159 106 265 0 Fr 87 87 136 87 223 0 Hg 80 80 121 78 201 +2 Xe 54 54 77 54 131 0 . Title: Protons, Neutrons, and Electrons Worksheet ...
Gas Chromatography-Mass Spectroscopy
cires1.colorado.eduMass Spectroscopy As the individual compounds elute from the GC column, they enter the electron ionization (mass spec) detector. There, they are bombarded with a stream of electrons causing them to break apart into fragments. These fragments can be large or small pieces of the original molecules.
MOSFET Device Physics and Operation
homepages.rpi.eduThe electrons enter and exit the channel at n+ source and drain contacts in the case of an n-channel MOSFET, and at p+ contacts in the case of a p-channel MOSFET. ... between the Fermi level and the top of the valence band. This is the case indicated for V = 0V in Figure 1.3(a). Carrier statistics tells us that the electron concentration then
KMBT 654-20131118121126 - Berger's Chemistry Class
bergerchem.weebly.comElectron Configurations Solutions Note: The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and …
4XDOLÛFDWLRQV - JABchem
jabchem.org.ukD The valence shell electron pair repulsion theory 3. X In the periodic table outlined above, one area is marked X. Moving across area X, from one element to the next, the extra electron usually occupies an orbital of type A s B p C d D f. 4. Which of the following molecules contains three atoms in a straight line? A BF 3 B CH 4 C H 2O D SF 6
Lewis Dot Structures - Missouri S&T
web.mst.eduThis theory is known as Valence Shell Electron Pair Repulsion Theory or VSEPR theory. The most common molecular shapes are shown in the table below. Bonding orbitals form when the valence orbitals on two atoms share a pair of electrons. The resulting orbital is called a “localized bonding orbital.” It is called “localized” because the ...
Particle Physics - University of Cambridge
www.hep.phy.cam.ac.ukElectron and Muon Currents Prof. M.A. Thomson Michaelmas 2011 125 •Here and matrix element • In handout 2 introduced the four-vector current which has same form as the two terms in [ ] in the matrix element • The matrix element can be written in terms of the electron and muon currents and • Matrix element is a four-vector scalar product – confirming it is Lorentz Invariant
Mass of the Electron - Michigan State University
web.pa.msu.eduproducing a visible beam along the electron track. The sphere is placed in a region where a magnetic field, ... except for one particular value of r. Thus, using this correction is approximate, and requires estimating an average value of s for the orbit. To do so will require a good sketch of the apparatus. ... The classic method for measuring ...
Atomic Mass and Atomic Number Worksheet Key
www.readington.k12.nj.usAtomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 sulfur …
Mole to Mole Answer Key - ISD 622
www.isd622.orgd. number of valence electrons involved in the reaction. In the reaction represented by the equation N2 + 3H2 2NH3, what is the mole ratio of nitrogen to ammonia? In the reaction represented by the equation 2A1203 —¥ 4Al + 302, what is …
Mass spectrometry practice questions - Weebly
www.sandsci.weebly.comElectron € € (b)€€€€ An atom of element Q contains the same number of neutrons as are found in an atom of 27A1. An atom of Q also contains 14 protons. (i)€€€€€ Give the number of protons in an atom of 27A1..... (ii)€€€€ Deduce the symbol, including mass number and atomic number, for this atom of element Q.
Interconnections: Silicides
web.stanford.eduIEEE Transaction Electron Dev., November, 1983. ... • As deposited films have high resistivity because they are amorphous or microcrystalline and therefore carrier mobility is low
พันธะเคมี (Chemical bond) - Maejo University
appliedchem.mju.ac.thทฤษฎี Valence-Shell Electron-Pair Repulsion (VSEPR) 23. 1. ทฤษฎีออร์บิทัลเชิงโมเลกุล (Molecular Orbital Theory) การรวมกนของอะตอมิกออร์บิทัลจะได้ โมเลคิวลาร์ออร์บิทัล .
Yield Analysis and Optimization - USI – Informatics
www.inf.usi.chFigure 1: An SEM (Scanning Electron Microscope) picture showing a bridging fault on Metal 3. Note the row of vias on each metal line. ... a very brief introduction is essential to understand flnal yield measurement at the foundry. ... their disposal. For example, with focused ion beam (FIB), existing circuit lines can be cut
EPC2023 – Enhancement Mode Power Transistor
epc-co.comGallium Nitride’s exceptionally high electron mobility and low temperature coefficient allows very low R DS(on), while its lateral device structure and majority carrier diode provide exceptionally low Q G and zero Q RR. The end result is a device that …
FinFET 3D Transistor & the Concept Behind It
microlab.berkeley.eduFinFET 3D Transistor & the Concept Behind It Chenming Hu, August 2011 1 Chenming Hu ... Y-K. Choi et al., IEEE Electron Device Letters, p. 254, 2000. Chenming Hu, August 2011 15. Silicon Body Needs to be <Lg/3. For good swing and device variation . ... Better mobility and
化合物半導体とエピタキシー
www.ccn.yamanashi.ac.jpHetero Bipolar Transistor(HBT) High Mobility Electron Transistor(HEMT) ...
La forma delle molecole - Zanichelli
online.scuola.zanichelli.itLa teoria VSEPR (Valence Shell Electron-Pair Repulsion, teoria della repulsio-ne delle coppie di elettroni del “guscio” di valenza) consente di ricavare la geometria, ossia la forma delle molecole, a partire dalle rappresentazioni delle formule di struttura di Lewis, partendo dal presupposto che le coppie
Supporting Information Unraveling the Dual Defect Sites in ...
www.rsc.orgThe room temperature electron paramagnetic resonance (EPR) spectra of the samples were measured by JOE L JES-FA200 spectrometer. The C and N ... absorbance at 350nm by UV –vis spectroscopy, from which the total amount of H 2O 2 produced during the reaction can be calculated.Figure S2 4 shows the standard curve
Coordination Chemistry III: Tanabe-Sugano Diagrams and ...
www.chem.uci.eduTo solve this problem we first need to determine the d-electron count for the [Co(NH3)6]2+ complex. Co NH 3 6 2 6NH 3 Co 2 So we have cobalt(II). Since cobalt is in the ninth column of the Periodic Table, it must be a d7complex so we can use the d7 Tanabe-Sugano diagram from the last slide. Next we need to find ∆o/B: O B
Electron Configurations Worksheet - Glendale Community …
web.gccaz.eduElectron Configurations Worksheet For atoms, the number of electrons = number of protons because atoms are neutral. Remember you are filling in ALL the electrons, not just valence, but ALL. The order of filling in electrons in the subshells is 1s 2s 2p 3s 3p 4s s subshells have only 1 orbital, 2 electrons per orbital = 2 electrons max
Electron affinity, Electronegativity, Ionization energy
www.chemie-biologie.uni-siegen.deElectron affinity, Electronegativity, Ionization energy 1. Electron affinity Definition: The energy released when an electron is added to a gaseous atom which is in its ground state to form a gaseous negative ion is defined as the first electron affinity. The …
Electron Configurations PowerPoint
www.birdvilleschools.netElectron Orbitals • Orbitals are clouds of probability within an energy level, so an actual orbital is a region of space, where an electron might be found. • Two orbital clouds are pictured below. There are more dots near the center of the picture, because an electron is most likely to be near the nucleus (center) of the atom (the electron
Electron Configurations, Orbital Notation and Quantum …
www.midlandisd.netthe set of quantum numbers for this valence electron are extremely easy to obtain. First, n = 3 since it is a 3p electron. Next it is a p electron and p sublevels have an ℓ value of 1. So far we know 3,1. To get the mℓ quantum number we go back to the orbital notation for the valence electron and focus on the 3p sublevel alone.
Electrons and Holes in Semiconductors
www.chu.berkeley.eduelectrons are of more interest to the operation of devices than valence electrons. An interesting thing happens when an electron breaks loose and becomes free. It leaves behind a void, or a hole indicated by the open circle in Fig. 1–5a. The hole can readily accept a new electron as shown in Fig. 1–5b. This provides another
Electron Configuration Practice Worksheet
www.mayfieldschools.orgElectron Configurations - Solutions Note: The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and that actinium (Ac) is the first element in the 5f block. If your periodic table doesn’t agree with this, your answers for elements near the f-orbitals may be slightly different.
Electron Current vs. Conventional Current
web.engr.oregonstate.eduElectron Current vs. Conventional Current In 1752, prior to electricity being identified with the electron, Ben Franklin chose a convention re-garding the direction of current flow. Franklin assumed that positive charge carriers flowed from positive to negative terminals. We now know this is incorrect. In metals, the charge carrier is the
Similar queries
Electrons, Valence, Levels, Sublevels, Orbitals, and Electrons, Orbitals, Orbital, Electron, Valence Shell Electron Pair Repulsion, Pair, 1. Semiconductor Materials & Physics, Mass, Shell, Electron repulsion, Beam, Structures and Molecule Geometries Worksheet, Valence electrons, Of the electron, VSEPR, Molecular geometry, Valence-Shell Electron-Pair Repulsion, Electron configurations, Principle, Working, Scanning Electron, Molecular, Geometry, Chapter 2 Semiconductor Heterostructures, Electrons and holes, Holes, Mo 42 42, Protons, Neutrons, and Electrons Worksheet, Gas Chromatography-Mass Spectroscopy, Lewis Dot Structures, Mass of the Electron, Using, Method, High, Mobility, Yield Analysis and Optimization, Scanning Electron Microscope, Introduction, Focused ion beam, Transistor, High electron mobility, FinFET 3D Transistor & the Concept Behind It, High Mobility Electron Transistor, Valence Shell Electron-Pair Repulsion, Electron paramagnetic resonance, Spectroscopy, Periodic, Electron Configurations, Orbital Notation and Quantum, Notation, Electrons and Holes in Semiconductors