Search results with tag "Molecules"
Moles, Molecules, and Grams Worksheet
www.nclark.netMoles, Molecules, and Grams Worksheet – Answer Key 1) How many molecules are there in 24 grams of FeF3? 1.28 x 10 23 molecules 2) How many molecules are there in 450 grams of Na2SO4? 1.91 x 10 24 molecules 3) How many grams are there in 2.3 x 1024 atoms of silver? 421 grams 4) How many grams are there in 7.4 x 1023 molecules of AgNO 3? 209 grams
Mole Calculation Worksheet - Everett Community College
www.everettcc.edu4.3 x 1022 molecules H 3 PO 4 x 1 mole H 3 PO 4 = 7.1 x 10-2 moles H 3 PO 4 6.022 x 1023 molecules H 3 PO 4 5) How many molecules are in 48.0 grams of NaOH? 48.0 molecules NaOH x 1 mole NaOH x 6.022 x 1023 molecules NaOH 40 g NaOH 1 mole NaOH = 7.23 x 1023 molecules NaOH
0.5 mole/liter x 180 grams/mole x 1 liter = 90 g …
www.deanza.eduHow many molecules of sucrose in that 1 liter of 0.5M sucrose solution? How does that compare to the amount of solute in the 0.5M glucose solution? 0.5 mole/liter x 1 liter x 6.023x1023 molecules/mole = 3.012x1023 molecules = same number of molecules How much of the 0.5M glucose solution is needed to provide 100 mg of glucose?
Chapter 2 Atoms, Molecules, and Ions
www2.chemistry.msu.eduammonia ammonia always has 3 H and 1 N. Atoms, Molecules, and Ions Law of Conservation of Mass ... • Green, trace amounts. Atoms, Molecules, and Ions Chemical Formulas The subscript to the right of the element tells the number of atoms of that element in the compound.
MO Diagrams for Diatomic Molecules
www.chem.uci.eduHeteronuclear Diatomic Molecules: CO In molecules with more than one type of atom, MOs are formed from AOs that have different energies. Consider CO: 2sa 2pa C 2sb 2pb C≡O O σ σ* π π* σ σ* Bonding orbitals get polarized towards oxygen Anti-bonding orbitals get polarized towards carbon HOMO is on carbon LUMO is on carbon too!
Lighter Than Air: Why Do Balloons Float?
kicp-workshops.uchicago.eduWe are so accustomed to swimming in air we forget that it is made of atoms and molecules (mostly nitrogen molecules, N 2), that have mass. This fact means that when the air molecules are displaced by an object, they push back with a buoyant force equal to the mass of the volume air pushed aside. Materials: • 12 small sheets thin tissue paper
PHYSICAL AND CHEMICAL CHANGES - University of Tennessee
extension.tennessee.eduatoms while the O2 represents two oxygen atoms. Hydrogen peroxide molecules are very unstable and naturally decompose into water and oxygen gas. The chemical equation for this decomposition is: 2 H2O2 --> 2 H2O + O2. The equation above represents two hydrogen peroxide molecules decomposing into two water molecules and one oxygen molecule.
AP Biology Photosynthesis Chapter 8 Reading Guide ANSWER …
ma02212418.schoolwires.netof carbons is 3, or one net G3P molecule. 20. Three turns of the Calvin cycle nets one G3P because the other five must be recycled to RuBP. Explain how the regeneration of RuBP is accomplished. The carbon skeletons of 5 molecules of G3P are rearranged into 3 molecules of RuBP. This reconfiguration requires the energy of 3 ATP molecules. 21.
Chapter 3: Biological Molecules - WOU
people.wou.eduChapter 3: Biological Molecules What Are Lipids? • Molecules composed almost entirely of carbon and hydrogen with non-polar carbon-carbon bonds (Hydrophobic) Types of Lipids: 1) Oils & Fats: • Composed of carbon, hydrogen, and oxygen Form Chains: Function: Energy Storage 3 fatty acid sub-units (CH 2 w/ COOH) & Glycerol Fats / Oils = 9.3 ...
Mole Conversions Worksheet
www.gardencity.k12.ny.us12. Find the mass, in grams, of 1.00 x 1023 molecules of N2. 13. How many particles are there in 1.43 g of a compound with a gram formula mass of 233 g? 2414. How many grams are there in 3.4 x 10 molecules of NH 3? 15. Aspartame is an artificial sweetener that is 160 times sweeter than sucrose (table sugar) when dissolved in water.
What causes the aurora? - NASA
pwg.gsfc.nasa.govenergy to oxygen and nitrogen molecules, making them excited. When the molecules return to their normal state, they release photons, small bursts of energy in the form of light. When billions of these collisions occur and enough photons are released, the oxygen and nitrogen in the atmosphere emit enough light for the eye to detect them.
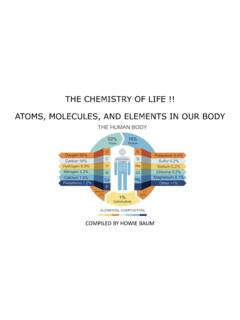
THE CHEMISTRY OF LIFE !! ATOMS, MOLECULES, AND …
www.uc.educleaning process, that only the H2O molecules are left - That means that there are no specks of dirt, salts, minerals or even viruses present in the water. While great for semiconductors, this is exactly the property that makes it harmful for humans. If ingested, it gets right to work and starts to absorb all the valuable minerals present in ...
Section 6 Ocean Primary Production
oceanexplorer.noaa.govcarbon to synthesize sugars and new water molecules. Photosynthesis gives off oxygen gas as a by-product, while chemosynthesis produces a wide variety of by-products, depending on what chemical substrate is used. The sugars produced provide both metabolic energy and substrate for synthesis of other biochemical molecules.
What is Ozone? Timeline of Stratospheric Ozone Depletion ...
www.nasa.govan additional factor that destroys ozone. CFCs are molecules made up of chlorine, fluorine and carbon. Because they are extremely stable molecules, CFCs do not react with other chemicals in the lower atmosphere, but exposure to ultraviolet radiation in the stratosphere breaks them apart, releasing chlo-rine atoms.
Bond dissociation energies in simple molecules
nvlpubs.nist.govA1110 2 145^03 jofStandards NATL 'VmmiiiSnSKBSllM^^'Wr 0 ' Admin.Bldg. All102145903 1970 QcToO S U573V31:1970C.1NBS-PUB-C 1964 NSRDS-NBS31 BondDissociationEnergies InSimpleMolecules U.S.DEPARTMENTOFCOMMERCE …
A level Biological Molecules practise OCR ExamBuilder
revise4science.weebly.comBiology A A level Biological Molecules practise Amy Vickers Please note that you may see slight differences between this paper and the original. Candidates answer on the Question paper. OCR supplied materials: Additional resources may be supplied with this paper. Other materials required: • Pencil • Ruler (cm/mm) Duration: Not set
3 ATOMS AND MOLECULES - National Institute of Open …
nios.ac.inSCIENCE AND TECHNOLOGY Atoms and Molecules 52 Notes MODULE - 2 Matter in our Surroundings Table 3.1 : Relative sizes Radius (in m) Example 10 10 Atoms of hydrogen 10 4 Grain of sand 10 1 Water melon 0.2×10 1 Cricket ball You can not see atoms with your naked eyes but by using modern techniques, we
Science Class 9 Notes – Atoms and Molecules - NCERT help
ncerthelp.com[The molecules of an element is made up of only one and same type of atoms, while the molecule of a compound is made up of dissimilar atoms] 7. Atomicity : The number of atoms present in a molecule of an element or a compound is known as its atomicity. e.g. the atomicity of oxygen is 2 while atomicity ozone is 3. 8. Ion :
Covalent BondingCovalent Bonding - Weebly
www.schoolisinsession.weebly.commolecules. Explain why their shapes differ. PF 3 is trigonal pyramidal with sp3 hybrid orbitals. PF 5 is trigonal bipyramidal with sp3d hybrid orbitals. Shape is determined by the type of hybrid orbital. 67. List in a table, the Lewis structure, molecular shape, bond angle, and hybrid orbitals for molecules of CS 2, CH 2 O, H 2 Se, CCl 2 F 2 ...
Lecture 2 Hamiltonian operators for molecules
www.southampton.ac.ukLecture 2 Hamiltonian operators for molecules C.-K. Skylaris CHEM6085: Density Functional Theory CHEM6085 Density Functional Theory. The (time-independent) Schrödinger equation is an eigenvalue equation operator for property A eigenfunction eigenvalue Energy …
Introduction to GaussView and Gaussian
comp.chem.umn.eduwww.msi.umn.edu Gaussian 03: an electronic structure package capable of predicting many properties of atoms, molecules, and reactive systems e.g. utilizing ab initio, density functional theory, semi-empirical, molecular mechanics, and hybrid methods.
BIOLOGY Grade 9 - Science
msschultzteaching.weebly.comSection 7.3, Eukaryotic Cell Structure. MiniLab 7.1, Measuring Objects Under A Microscope, ... Biology - Grade 9 . Molecules to Organisms: Cells –Structures and Functions, Levels of Organization ... Reinforcement and Study Guide, Chapter 7, A View of the Cell, p.29-32. Chapter 8, Cellular Transport and Cell Cycle
AQA AS Biology 3.1 Biological Molecules - QCL Science
qclscience.weebly.comalevelbiology.co.uk 6 5. The figure below shows the effect of increasing the concentration of the substrate (PABA) on the rate of reaction. Curve A shows the rate of reaction without the presence of the competitive inhibitor sulfonamide.
Greenhouse Gases CHAPTER 4 - University of Chicago
forecast.uchicago.eduIn fact, most of the gases in the atmosphere do not absorb or emit IR light at all, because vibrations in their bonds do not create an imbalance in the electrical field. Both O 2 and N 2, the most abundant gases in the atmosphere, are symmetric molecules, made of two identical atoms whose electric fields just cancel each other out.
ASAP 2020 - Micromeritics
www.micromeritics.comallows only molecules of desired sizes to enter and leave, creating a selec-tive catalyst that will produce ... microporosity are used to predict the capacity of a material to store hydrogen. ... with widely differing isotherm shapes. • The patented Smart Dosing™ routine
Contrails - NASA
www.nasa.govit is reflected by the water molecules within them, making the cloud visible and distinguishable from its background, the sky. Img. 4. An F/A-18 Hornet in flight (Photo courtesy of the United States Navy) Img. 5. An F-35 departing Elgin Air Force Base, Florida (Photo courtesy of the United StatesAir Force) Img. 3. Persistent contrail (Photo ...
LEWIS STRUCTURES General Rules for Drawing Lewis Structures
clas.sa.ucsb.edu7. In drawing Lewis structures for relatively small molecules and polyatomic ions, the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms. EXAMPLE: Write the Lewis structure for CH2O where carbon is the central atom. Step 1: Determine the total number of electrons available for bonding.
Covalent Bonding
www.simplychemistry.orgeXtension create a Frayer model for each of the terms covalent bond, diatomic molecule, molecular compound, and molecular formula. Lesson summary molecules and molecular compounds The electrons in a molecular compound are shared. The atoms in a molecular compound are held together by covalent bonds.
E-Cigarette, or Vaping, Products Visual Dictionary
www.cdc.govin many shapes, sizes, and colors. ... Synthetic cannabinoids are a class of synthetic molecules that bind to cannabinoid receptors in the brain and body (the same receptors to which THC and CBD attach). They are “designer drugs” that are usually smoked and have been marketed as
Introduction to Light and Color - NASA
www.nasa.govor ink is formed by pigment molecules present in flowers, trees, and animals. The color is made by absorbing, or subtracting, certain parts of the spectrum and reflecting or transmitting the parts that remain. Each pigment molecule seems to have its own distinct characteristic way of reflecting, absorbing, or transmitting certain wavelengths.
More Is Different - KIT
www.tkm.kit.edupyramidal molecules, made of heavier atoms. Hydrogen phosphide, PH3, which is twice as heavy as ammonia, inverts, but at one-tenth the ammonia frequency. Phosphorus triRuoride, PF3, in which the much heavier fluorine is substituted for hydrogen, is not observed to invert at a measurable rate, although theo-
Protein Precipitation for Biological Fluid Samples Using ...
www.agilent.comprotein molecules, and significantly lowers the solubility of the proteins, resulting in proteins precipitating out. ... Because biological fluids are more viscous, sample aliquoting can be challenging. Pipetting is the method of choice for accurately transferring desired
Molecular energy levels and spectroscopy
vallance.chem.ox.ac.ukDiatomic molecules are often approximated as rigid rotors, meaning that the bond length is assumed to be fixed. Solving the Schrodinger equation for a rigid rotor gives the following energy levels: E(J) = B J(J+1) In this equation, J is the quantum number for total rotational angular momentum, and B is the rotational
MOS Capacitor - Chenming Hu
www.chu.berkeley.edu1.5 nm. One nanometer is equal to 10 Å, or the size of a few oxide molecules. Before 1970, the gate was typically made of metals such as Al (hence the M in MOS). After 1970, heavily doped polycrystalline silicon (see the sidebar, Three Kinds of Solid, in Section 3.7) has been the standard gate material because of its ability to
Regents Review: Cells & Cell Transport
tagscience.weebly.com3.What is a similarity between all bacteria and plants? A)Any cell produced from this skin cell will have the same ... synthesis of molecules D)release of energy 27.What is the main function of a vacuole in a cell? A)1 B)2 C)3 D)4 ... 50.The diagram below represents a section of a plasma membrane. What does structure X represent? A)synthesis of ...
KM 654e-20161208145634
npalayman.weebly.comThe VSEPR (Valence Shell Electron Pair Repulsion) Theory helps predict the shapes of molecules and is based on the premise that electrons around a central atom repel each other. Electron domains are areas of high electron density such as bonds (single, double or triple) and lone-pairs of electrons. In simple
Multiple Choice Questions - NCERT
www.ncert.nic.in(a) Atoms are not able to exist independently (b) Atoms are the basic units from which molecules and ions are formed (c) Atoms are always neutral in nature (d) Atoms aggregate in large numbers to form the matter that we can see, feel or touch 3. The chemical symbol for nitrogen gas is (a) Ni (b) N 2 (c) N+ (d) N 4. The chemical symbol for sodium is
AAATOMSTOMSTOMS ANDANDAND M M MOLECULES - …
www.ncert.nic.in(ii) barium chloridesodium sulphate 1.22 g 1.53 g (iii) lead nitrate sodium chloride 2.07 g 1.17 g • Prepare separately a 5% solution of any one pair of substances listed under X and Y each in 10 mL in water . • Take a little amount of solution of Y in a conical flask and some solution of X in an ignition tube. • Hang the ignition tube in ...
A-Level Biology Question and Answers 2020/2021
www.s-cool.co.ukBiological Molecules and Enzymes (Answers) Answer outline and marking scheme for question: 1 Give yourself marks for mentioning any of the points below: a) (i) Amino acid. (ii) Possession of CH 3 group/different R group. (2 marks) b) (i) Glycogen consists of glucose/one type of monomer. Many different amino acids combine to form proteins.
Equation Stoichiometry Chemistry 110
www.cerritos.edud. How many grams of oxygen are needed to react with 7.22 x 10 . 24. molecules of C. 8. H. 18? 7.22 x 1024molec. C8H18 X . 1mol C8H18 6.02x1023molec X 25mol O2 2mol C8H18 X 32.0g O2 1mol O2 = 4.80 x 103g O2. Answer _____ 2] How many grams of aluminum oxide are formed when 25.0 grams of Aluminum are reacted with oxygen gas? a. Write the balanced ...
C.V. Raman Global University, Bhubaneswar
cgu-odisha.ac.inUnit 1: Structure and Bonding Schrödinger equation, interpretation of wave functions, molecular orbital theory of diatomic molecules, nomenclature and isomerism in coordination complex. Crystal field theory and the energy level diagrams for transition metal ions and their magnetic properties Unit 2: Phase rule and Solid State
Chapter 5 - Atmospheric Moisture - Texas Tech University
www.atmo.ttu.eduthat can exist in the atmosphere as a vapor. 2 ATMO 1300 Evaporation and Condensation • Evaporation – Change in phase from liquid to vapor. The process in which molecules break free of the liquid volume. • Condensation – Change in phase from vapor to …
Science Starts With a Question
ssec.si.eduScience is fun but can also be dangerous. Emphasize safety ... In a chemical process, the atoms that make up the original substances are regrouped into di!erent molecules, and these new substances have di!erent properties from those of the reactants. The total number of each
Questions with Answers- Lipids
med.fau.edu20._____ Which is a characteristic of the lipids in a biological membrane? a) Specific glycerophospholipids are distributed equally on the two membrane surfaces. b) Lipid molecules are held in fixed positions by non-covalent bonds with proteins. c) The fluidity of the membrane decreases with lower levels of saturated fatty acids.
Molecules of the Atmosphere
butane.chem.uiuc.edu3) break when the molecules absorb ultraviolet radiation. Re-forming those bonds releases heat energy so the temperature increases with altitude in this layer. The troposphere is the region of the atmosphere closest to the Earth and is the region of all weather events. This layer is heated by the surface of the Earth, which in turn is heated by ...
Molecules on Surfaces - University of Chicago
psec.uchicago.eduPumps for Vacuum Systems • High Vacuum. 2-stage pumping required. Generally no bake-out. Roughing pump backing: - diffusion pump - turbo pump - cryopump. 10-5 . to 10-8 . torr • Ultra-High Vacuum. 3-stage pumping required. Bake-out is essential. Roughing pump backing: - turbo pump - cryopump. Ion pump operating alone after bake-out. 10-9 ...
Lewis Dot Structures and Molecule Geometries Worksheet ...
www.teachengineering.org—Lewis Dot Structures and Molecule Geometries Worksheet Answer Key 1 Lewis Dot Structures and Molecule Geometries Worksheet Answer Key How to Draw a Lewis Dot Structure ... Look up the electronegativity values for each element in your structure. The least electronegative atom represents the central atom. Hydrogen is the only exception to this ...
Essential IND Strategies: Fundamental Considerations on ...
health.ucdavis.edudrug formulations, with well characterized impurity profiles. 5) ... Does not address potential genotoxic impurities in API Genetic Toxicology . Requirements ICH Core Battery: ... Small molecule – commonly stand alone studies Biological – …
Similar queries
Moles, Molecules, and Grams Worksheet, Molecules, Molecules 3, Worksheet, Everett Community College, Ammonia ammonia, Green, Atoms and molecules, Atoms, 3 molecules, Atmosphere, Section, Primary Production, A level Biological Molecules, OCR ExamBuilder, Biology, Covalent BondingCovalent Bonding, Shapes, Lecture 2 Hamiltonian operators for molecules, Introduction to GaussView and Gaussian, Study Guide, Biological Molecules, Greenhouse Gases, ASAP 2020, Predict, Contrails, NASA, Diatomic, Vaping, Products Visual Dictionary, More Is Different, Biological, Diatomic molecules, Section 3, Review, Cell, VSEPR, Valence Shell Electron Pair Repulsion, Predict the shapes of molecules, Sulphate, A-Level, Grams, Unit, Bonding, Science Starts With a Question, Molecules of the Atmosphere, Pumps, Turbo, Structures and Molecule Geometries Worksheet, Structures and Molecule Geometries Worksheet Answer Key, Your, Drug, Genotoxic impurities, Small molecule