Search results with tag "Impurities"
A C DNA R (M P L P C R M7(R1) - ICH
database.ich.orgdegradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2): Impurities in New Drug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive.
ICH HARMONISED TRIPARTITE GUIDELINE
database.ich.orgImpurities are an important class of potential drug substance CQAs because of their potential impact on drug product safety. For chemical entities, impurities can include organic impurities (including potentially mutagenic impurities), inorganic impurities e.g., metal residues, and residual solvents (see ICH Q3A and Q3C). For
476 Organic Impurities in Drug Substances and Drug …
www.usp.orgBRIEFING 476 Organic Impurities in Drug Substances and Drug Products. As part of an ongoing monograph modernization initiative, the United States Pharmacopeial Convention (USP) is updating general chapter Impurities in Drug Substances and Drug Products 1086 and proposing this new chapter that addresses organic impurities testing for articles subject
Q3C (R6) Step 5 - impurities: guideline for residual solvents
www.ema.europa.eusolvent may be based on concepts in this guideline or the concept of qualification of impurities as expressed in the guideline for drug substance (Q3A, Impurities in New Drug Substances) or drug product (Q3B, Impurities in New Drug Products), or all three guidelines. 2. Scope of the guideline
Introduction to ICH - The Quality Guidelines – An Overview
admin.ich.orgICH Q 3 – Impurities A set of three guidelines addressing the chemistry and safety aspects of impurities, including the listing of impurities in specifications. Defines the thresholds for reporting, identification and qualification of impurities in API and finished product. Specific guideline on residual solvents
Q3C (R5) Impurities: guideline for residual solvents
www.tga.gov.auqualification of impurities as expressed in the guideline for drug substance (Q3A, Impurities in New Drug Substances) or drug product (Q3B, ... Non-genotoxic animal carcinogens or possible causative agents of other irreversible toxicity such as neurotoxicity or teratogenicity.
Chemistry, Manufacturing, and Controls of Drug Candidates ...
accelerate.ucsf.eduGenotox Impurities* •“exposure to the potentially genotoxic impurities can not exceed 60 micrograms per day. For longer duration clinical trials the levels would have to be further reduced; for clinical trials of greater than one year duration, the daily exposure to these impurities should not exceed 1.5 micrograms.”
1086 IMPURITIES IN DRUG SUBSTANCES AND DRUG …
www.uspnf.comcontainer–closure system, inorganic/elemental impurities, and residual solvents are out of the scope of this chapter. USP42 Communications The regulatory and compendial standards for the control of impurities continue to evolve due to advancements in analytical science, technology, and toxicology. Therefore, communications USP42
Genotoxic Impurities and Its Risk Assessment in Drug …
lupinepublishers.comGenotoxic impurities can get incorporated into drug substances through the various sources, the major source is the starting material used in the synthesis of drug substances and its impurities. Similarly, genotoxic intermediate and by-products formed in the
Q3D(R2) - ICH
database.ich.org1 Appendix 2: Established PDEs for Elemental Impurities 2 Table A.2.1: Permitted Daily Exposures for Elemental Impurities 1 Element Class 2 Oral PDE µg/day Parenteral PDE, µg/day Inhalation PDE, µg/day Cd 1 5 2 3 Pb 1 5 5 5 As 1 15 15 2 Hg 1 30 3 1 Co 2A 50 5 3 V 2A 100 10 1 Ni 2A 200 20 65
Q3D(R1) - ICH
database.ich.orgElemental impurities in drug products may arise from several sources; they may be residual catalysts that were added intentionally in synthesis or may be present as impurities (e.g., through interactions with processing equipment or container/closure systems or by being present in components of the drug product). ...
Annexes to: CPMP/ICH/283/95 Impurities: Guideline for ...
www.ema.europa.eusolvents in active substances . Specifications for class 1 solvents In both the ICH and VICH guidelines on impurities: residual solvents it is stated that “ solvents in class 1 should not be employed in the manufacture of drug/active substances, excipients, and
Lessons learnt from presence of N-nitrosamine impurities ...
www.ema.europa.euLessons learnt from presence of N-nitrosamine impurities in sartan medicines . Overview and recommendations . Overview . In mid-2018, the European medicines regulatory network. 1. became aware of the presence of . N-nitrosamines in sartan. 2. active pharmaceutical ingredients (APIs) and instituted regulatory action s
Q3C (R8): Impurities: guideline for residual solvents
www.ema.europa.euQ3C (R8): Impurities: guideline for residual solvents Step 2b Transmission to CHMP 30 April 2020 Adoption by CHMP 30 April 2020 Release for public consultation 4 May 2020 Deadline for comments 30 July 2020 Comments should be provided using this template. The completed comments form should be sent to ich@ema.europa.eu
厚生労働省医薬・生活衛生局医薬品審査管理課長 ( 公 印 省 …
www.mhlw.go.jpAssessment report:Nitrosamine impurities in human medicinal products. Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products. FDAのガイダンス注
医薬品の元素不純物ガイドライン(ICH Q3D)と ICP-MS:分 …
www.kanto.co.jpにはUSPから<232> Elemental Impurities-Limitsおよび <233> Elemental Impurities-Proceduresがドラフトとし て提案された2,3)。このことを受け、2009年11月にICHの新たな トピックスとしてQ3D(元素不純物)が決定された。なお、EMA の提案は、後の2012年に、欧州薬局方(EP: The European
Ch. 26: Gravimetric Analysis
chem320.cs.uwindsor.careacting with the precipitant. e.g., In the gravimetric analysis of Be2+, Mg2+, Ca2+ or Ba2+ with the reagent N-p-chlorophenylcinnamohydroxamic acid, impurities such as Ag+, Mn2+, Zn2+, Cd2+, Hg2+, Fe2+, and Ga3+ are kept in solution by excess KCN. Post-precipitation: Impurities collect on the pure precipitated product while it is standing in the
CPMP/ICH/283/95 ICH Topic Q3C (R4) Impurities: Guideline ...
www.tga.gov.auICH Topic Q3C (R4) Impurities: Guideline for Residual Solvents Page 4/22 This guideline does not apply to potential new drug substances, excipients, or drug products used during the clinical research stages of development, nor does it apply to existing marketed drug products. The guideline applies to all dosage forms and routes of administration.
Q3C (R8) Step 5 - impurities: guideline for residual solvents
www.ema.europa.euStep 5 . Transmission to CHMP 30 April 2020 Adoption by CHMP 30 April 2020 Release for public consultation 4 May 2020 Deadline for comments 30 July 2020 Final adoption by CHMP 20 May 2021 Date for coming into effect 20 November 2021 . ICH guideline Q3C (R8) on impurities: guideline for residual solvents
Journal of Pharmaceutical and Biomedical Analysis
www.almacgroup.comUSP required elemental impurities (As, Cd, Hg, Pb, V, Cr, Ni, Mo, Cu, Pt, Pd, Ru, Rh, Os and Ir) in a single analysis. The matrix was used in the validation of a method to determine elemental impurities in TP-6076 active pharmaceutical ingredient (API) by ICP-MS according to the procedures defined in USP 233 and to GMP requirements.
Q&A on the CHMP Guideline on the Limits of Genotoxic ...
www.ema.europa.eupotentially genotoxic impurities or to higher levels of previously recognized potentially genotoxic impurities then the situation should be discussed with the competent authority. Question 9. What is a reasonable policy for setting specifications for potentially genotoxic
EI Risk Assessment - European Medicines Agency
www.ema.europa.euICH Q3D Guideline for Elemental Impurities – Practical Implementation of ICH Q3D • ICH Q3D recommends taking a . risk based approach. • Focus is on the . final product – the fishbone diagram assists by advising on the components for consideration: all. potential sources of elemental impurities should be considered and evaluated for their
ICH Q2B Guideline Validation of Analytical Procedures ...
www.bioagilytix.comFeb 12, 2016 · The ICH guideline on validation has been succeeded by the ICH guidelines on Impurities in New drug substances and Drug Products. There have been threshold levels defined for • Reporting thresholds • Identification thresholds They should be applied instead of quantitation and detection limits. 5. Range Analytical procedure Range
Melting Point Determination
www.thinksrs.combecause even small quantities of impurities change the melting point, or at least clearly enlarge its melting range. Melting point determinations are more than just a classroom exercise in the organic chemistry lab. The test is still an important technique for gauging purity of organic and pharmaceutical compounds.
Guideline on assessment and control of DNA reactive ...
www.ema.europa.euof DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk which was used as a template, with amendments introduced in order to cover the issues specific to VM Ps. 2. Scope of guideline This document is intended to provide guidance for new veterinary drug substances and new VMPs,
VALIDATION OF ANALYTICAL P TEXT AND METHODOLOGY …
database.ich.orgREQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY Q2(R1) ... - Testing for impurities can be either a quantitative test or a limit test for the impurity in a sample. Either test is intended to accurately reflect the purity
Guideline on Inhalational medicinal products
www.ema.europa.euimpurities, process validation, stability testing, specifications) as well as safety and efficacy aspects, are described in other guidance documents, including ICH guidelines. Detailed guidance on pharmaceutical development study designs (e.g., priming studies) and the analytical procedures used primarily for inhalation and nasal products (e.g ...
Procedure under Article 5(3) of Regulation EC (No) 726 ...
www.ema.europa.euCommittee for Medicinal Products for Human Use (CHMP) Assessment report . Procedure under Article 5(3) of Regulation EC (No) 726/2004 . Nitrosamine impurities in human medicinal products . Procedure number: EMEA/H/A-5(3)/1490 . Note: Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted.
Annex 9 Guide to good storage practices for pharmaceuticals
www.who.int• The stability testing of pharmaceutical products containing ... The undesired introduction of impurities of a chemical or microbio-logical nature, or of foreign matter, into or onto a starting m aterial, or intermediate or finished product during production, sampling, pack-
Quality Control of Compounded Radiopharmaceuticals
pharmacyce.unm.eduPITFALLS IN DETERMINATION OF RADIOCHEMICAL PURITY 2,13,28-30 ... PRINCIPLE OF RADIOCHEMICAL PURITY ANALYSIS Radiochemical purity (RCP) of a radiopharmaceutical is defined as the percent of the total ... impurities, other than the hydrolyzed-reduced impurity, ...
Q3B(R2) - ICH
database.ich.orgguideline “Residual Solvents” should also be consulted, if appropriate. 1.3 Scope of the guideline This guideline addresses only those impurities in new drug products classified as degradation products of the drug substance or reaction products of the drug substance
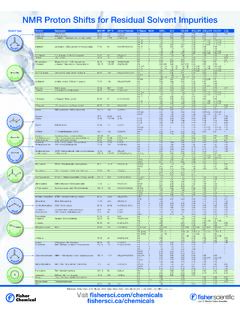
NMR Proton Shifts for Residual Solvent Impurities
beta-static.fishersci.comChloroform Trichloromethane / Formyl trichloride 119.38 61 CHCl 3 CH s 7.26 – 7.88 8.32 8.02 7.58 6.15 1,2-Dichloroethane EDC / Ethylene dichloride / Glycol dichloride 98.96 81-85 ClCH
ICH M7: Assessment and Control of Mutagenic Impurities - …
pqri.orgM7 to new marketing applications that do not include Phase 2B/3 clinical trials is not expected until 36 months after ICH publication of M7 (e.g., new dosage forms, or new DMFs supporting generic drug applications, may follow pre-M7 guidance until July 2017). • The 36 month implementation period is also
Temperature Dependence of Semiconductor Conductivity
www.iiserkol.ac.incharged impurities. As a result, as the temperature decreases, impurity scattering increases, and the mobility decreases. This is just the opposite of the effect of lattice scattering. The total mobility then is the sum of the lattice-scattering mobility and the impurity-scattering mobility.
The Investigational New Drug (IND) and New Drug ...
ocw.jhsph.eduFeb 02, 1998 · The Investigational New Drug (IND) and New Drug Application (NDA) Process Susan Honig, MD ... and strength of the drug • Impurities, sterility • Product consistency. Requirements for a New IND: ... • 3 products converted from accelerated approval to full approval – Taxotere, with expansion of its indication ...
Essential IND Strategies: Fundamental Considerations on ...
health.ucdavis.edudrug formulations, with well characterized impurity profiles. 5) ... Does not address potential genotoxic impurities in API Genetic Toxicology . Requirements ICH Core Battery: ... Small molecule – commonly stand alone studies Biological – …
STABILITY TESTING OF ACTIVE SUBSTANCES AND …
www.who.intpharmaceutical product can be established, and storage conditions can be recommended. ... are named under the headings “purity tests” and/or “section on impurities”. b) For active substances not described in an official pharmacopoeial monograph, there are two options:
SOIL STABILIZATION METHODS AND MATERIALS
www.diva-portal.orgpresence of foreign matters or impurities water-cement ratio curing temperature presence of additives specific surface of the mixture. Depending on factor(s) involved, the ultimate effect on setting and gain in strength of cement stabilized soil may vary. Therefore, this shouldbe taken into account during mix
FDA Expectations for Toxicology Support of Clinical Trials ...
www.expedient-solutions.com• Impurities B and C must be identified and reported (>0.1%) • Impurity A must also be reported (>0.5%) – use HPLC RT • Impurity B & C – must be evaluated for mutagenicity using “in silico tests” – If positive – do Ames test – If Ames test positive – control as a …
NMR Chemical Shifts of Trace Impurities: Common …
kgroup.du.eduethylene, propylene, and carbon dioxide) often encoun- ... carbonate,dimethylcarbonate,dimethylmalonate,furan, Apiezon H grease, hexamethylbenzene, hexamethyldisil-oxane, imidazole, pyrrole, and pyrrolidine), have also beenaddedtothisexpandedlist. Experimental Section All deuterated solvents were obtained …
London, 28 June 2006 EMEA/CHMP/QWP/251344/2006 …
www.ema.europa.eufor class 2 solvents in the Q3C Note for Guidance on Impurities: Residual Solvents. This approach calculates a “Permitted Daily Exposure” (PDE), which is derived from the NOEL, or the lowest-observed effect level (LOEL) in the most relevant …
Nitrosamines EMEA-H-A5(3)-1490 - Information on ...
www.ema.europa.euimpurities if amines (see examples above) are present in any step of the synthesis. ... Step 2 confirmatory testing: in the event that a risk of presence of nitrosamines is identified as a result of the risk evaluation, confirmatory testing should be carried out using appropriately
Implementation Process Article 5(3) Nitrosamine
www.ema.europa.eutriggered by the EMA Executive Director (ED) requesting the CHMP to conduct a scientific evaluation on the presence of nitrosamine impurities in human medicines containing chemically synthesised active pharmaceutical ingredients (APIs). The procedure was foreseen to run in a 2-step approach as follows:
IMPURITIES IN EW DRUG SUBSTANCES Q3A(R2) - ICH
database.ich.orgimpurities found in batches manufactured by the proposed commercial process. Those individual impurities with specific acceptance criteria included in the specification for the new drug substance are referred to as "specified impurities" in this guideline. Specified impurities can be identified or unidentified.
IMPURITIES IN EW DRUG SUBSTANCES Q3A(R2)
database.ich.orgImpurities in New Drug Substances 3 inorganic impurities in the new drug substance specification should be discussed. Acceptance criteria should be based on pharmacopoeial standards or known safety data. 3.3 Solvents The control of residues of the solvents used in the manufacturing process for the new
IMPURITIES GUIDELINE FOR RESIDUAL S Q3C(R8) - ICH
database.ich.org1 PART I: IMPURITIES: GUIDELINE FOR RESIDUAL SOLVENTS Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 17 July 1997, this Guideline is recommended for adoption to the three regulatory parties to ICH 1. INTRODUCTION The objective of this guideline is to recommend acceptable amounts for residual solvents in
Impurities in Drug Products and Drug Substances - A USP ...
latam-edu.usp.orgDr. Ravichandran’s industrial experience includes several years in the diagnostics and pharmaceutical industry where he gained experience in analytical methods development for both raw materials and finished product characterization, methods ... industry to detect nitrosamine impurities in ARB drug substances and drug products.
IMPURITIES GUIDELINE FOR RESIDUAL SOLVENTS Q3C(R6)
database.ich.orgICH HARMONISED GUIDELINE. IMPURITIES: GUIDELINE FOR RESIDUAL SOLVENTS. Q3C(R6) Final version . Adopted on 20 October 2016. This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process.
Similar queries
IMPURITIES, Guideline, Residual, Organic Impurities in Drug Substances and Drug, Organic Impurities in Drug Substances and Drug Products, Impurities in Drug Substances and Drug Products, Introduction to ICH - The Quality Guidelines – An Overview, Genotoxic, Genotoxic impurities, Elemental impurities, Parenteral, Substances, Active pharmaceutical, Guideline for residual, Nitrosamine impurities in human medicinal products, Elemental Impurities-Procedures, Analysis, ICH Topic Q3C (R4) Impurities: Guideline, Residual Solvents, Step 5, Impurities: guideline, Journal of Pharmaceutical and Biomedical Analysis, DETERMINATION, Purity, Impurities in pharmaceuticals, VALIDATION OF ANALYTICAL, PHARMACEUTICALS, Testing, For Human, Good storage practices for pharmaceuticals, Quality Control of Compounded Radiopharmaceuticals, PURITY ANALYSIS, Proton Shifts for Residual Solvent Impurities, Chloroform, Assessment and Control of Mutagenic Impurities, Effect, New drug, DRUG, 3 products, Small molecule, OF ACTIVE SUBSTANCES AND, Pharmaceutical, FDA Expectations for Toxicology Support of Clinical Trials, Propylene, Carbonate, Implementation, Evaluation, Impurities in New Drug, IMPURITIES GUIDELINE FOR RESIDUAL, IMPURITIES: GUIDELINE FOR RESIDUAL, IMPURITIES GUIDELINE