Chemistry Of Life
Found 7 free book(s)THE CHEMISTRY OF LIFE !! ATOMS, MOLECULES, AND …
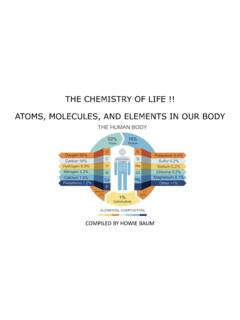
www.uc.eduCarbon-Based life form !! Carbon is a key component of all known life on Earth, representing approximately 45-50% of all living things, such as animals and plants. Complex molecules are made up of carbon bonded with other elements, especially oxygen and hydrogen and frequently also with nitrogen, phosphorus and sulfur.
Nuclear Chemistry Practice Problems
ion.chem.usu.eduChemistry 1110 – Chapter 5 – Nuclear Chemistry – Practice Problems Page | 10 47. The half-life of a radioisotope is A) one-half of the time it takes for the radioisotope to completely decay to a nonradioactive isotope. B) the time it takes for the radioisotope to become an isotope with one-half of the atomic
Syllabus Cambridge IGCSE Chemistry 0620
www.cambridgeinternational.orgChemistry 0620. Why choose Cambridge? Cambridge Assessment International Education prepares school students for life, helping them develop an informed curiosity and a lasting passion for learning. We are part of the University of Cambridge.
Gas Laws Save Lives: The Chemistry Behind Airbags
www.chemistry.wustl.edupotentially life-saving decisions about using airbags. Overview of How Airbags Work Timing is crucial in the airbag's ability to save lives in a head-on collision. An airbag must be able to deploy in a matter of milliseconds from the initial collision impact. It must also be prevented from deploying when there is no collision.
Kinetics formulas copy - Chemistry 301
ch301.cm.utexas.eduHalf-life (t 1/2): The time it takes for the concentration to drop to one half its current value during the course of the reaction. Note that the “current value” is typically the initial starting value - but not always. Rate Laws for: a A products (all the following equations assume that k is for the overall reaction)
AP Chemistry 2019 Free-Response Questions
apcentral.collegeboard.orgCHEMISTRY FREE-RESPONSE QUESTIONS . GO ON TO THE NEXT PAGE. -6-(c) Calculate the concentration of urea, in mol/L, in the saturated solution at 20.°C. (d) The student also determines that the concentration of urea in a saturated solution at 25°C is 19.8 . M. Based on this information, is the dissolution of urea endothermic or exothermic?
Material Safety Data Sheet Page 1 of 2 Vinegar
dept.harpercollege.eduDisclaimer: Scholar Chemistry and Columbus Chemical Industries, Inc., (“S&C”) believes that the inform ation herein is factual but is not intended to be all inclusive. The information relates only to the specific material designated and does not relate to its use in combination with other materials or its use as to any