Lewis Acids
Found 5 free book(s)Organic Chemistry Specific Name Reactions
www.meritnation.comNote: Aromatic carboxylic acids do not undergo Friedel-Crafts reaction because the carboxyl group is deactivating and the catalyst aluminium chloride (Lewis acid) gets bonded to the carboxyl group. Friedel-Crafts acylation reaction The reaction of benzene with an acyl halide or acid anhydride in the presence of Lewis acids (AlCl 3
Fundamentals of Protein Chemistry - Duke University
genome.duke.eduMolecular Biology of the Cell by Bruce Alberts, Dennis Bray, Julian Lewis, Martin Raff, Keith Roberts, and James D. Watson, Garland Publishing, NY 1994 . Histology by Bergman, R.A., Afifi A.K. and Heidger, P.M. Saunders Publishing, ... Determines the precise molar ratios of amino acids present. Can also be used to accurately determine ...
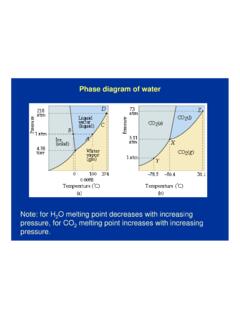
Phase diagram of water - Columbia University
www.columbia.eduLewis dot diagram O H 104.5o H space filling model. O-H bonds are polarized because of the difference in electronegativity between the O and H atoms. ... nucleic acids. “Hydrophobic effect”, or the exclusion of non-polar compounds is another unique property of water caused by
Reactions of Aromatic Compounds - Rutgers University
crab.rutgers.eduBut the addition of a strong Lewis acid (electron pair acceptor), such as FeBr 3, catalyses the reaction, and leads to the substitution product. The bromine molecule reacts with FeBr 3 by donating a pair of its electrons to the Lewis acid, which creates a more polar Br …
Chapter 11: Reactions of Alcohols - University of Northern ...
www2.unbc.caLewis acid (ZnCl2 )is needed to carry out the reaction. 3 oAlcohols react with HCl without the need for ZnCl2. The combination of HCl and ZnCl2is known as the Lucas reagent. 1 oalcohols follow and SN2 mechanism, 156