Pyramidal
Found 10 free book(s)Extrapyramidal symptom assessment Introduction
ifeet.orgFactors that may increase the risk of extrapyramidal symptoms in patients taking antipsychotic drugs include age, gender (female), race (black), psychiatric diagnosis, history
Treating Antipsychotic-Induced Extrapyramidal Symptoms ...
oshp.memberclicks.net4/11/2017 1 Treating Antipsychotic-Induced Extrapyramidal Symptoms with Vitamin B6 Cassandra Abeyta, PharmD | cassandra.abeyta@va.gov PGY 1 …
VSEPR and Molecular Shapes Tables
web.gccaz.eduAB5E: square pyramidal (central atom + 5 B atoms make 4-faced pyramid) – start with AB6 (octahedral) and replace one B atom with one lone pair – all six outer positions are identical, so any outer atom can be removed → square pyramidal shape – lone pair electrons push bonding electrons away → bond angles are now less than 90°
Trigonal Bipyramidal Electronic Geometry: AB5, AB4U, AB3U2 ...
www.chem.tamu.eduOne lone pair - square pyramidal 2. Two lone pairs - square planar |The lone pairs occupy axial positions because they are 90o from four bonding pairs. zResults in decreased repulsions compared to lone pairs in equatorial positions.
Lewis Structures, Shapes, and Polarity
www.everettcc.edutrigonal pyramidal, polar c. CCl 2 F 2 tetrahedral, polar d. CF 2 H 2 tetrahedral, polar e. CH 2 O trigonal planar, polar f. CHN linear, polar g. PI 3 trigonal pyramidal, polar h. N 2 O linear, polar i. SO 2 bent, polar j. CS 2 linear, non-polar k. CO linear, polar l. H 2 O bent, polar m. COF 2 trigonal planar, polar n. N 2 linear, non-polar o ...
Using VSEPR to Predict the Shapes of Molecules
www.angelo.edupyramidal <109.5° polar sp3 <109.5° 2 2 AX 2E 2 bent <109.5° polar sp 3 <109.5° 1 “Electron groups” include bonds, lone pairs, and odd (unpaired) electrons. A multiple bond (double bond or triple bond) counts as one electron group. 2 A multiple bond (double bond or triple bond) counts as one bond in the VSEPR model.
D-orbital splitting diagrams
www.cchem.berkeley.eduSquare pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d xz d z2 d x2-y d xy d yz d xz z x y. Title: Microsoft Word - Document1 Created Date: 5/17/2017 11:24:42 PM ...
Structure and Function of Neurons
assets.cambridge.orgPyramidal cells (depicted somewhat realistically in A and iconically in B) have a cell body shaped like a triangular pyramid, an extensively branched spiny apical dendrite, shorter basal dendrites, and a single axon emerging from the basal pole of the …
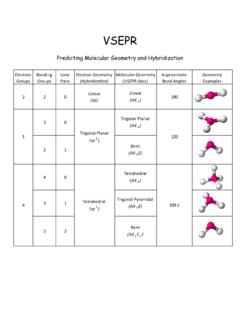
4 109.5 3 1 3 (AX 3 VSEPR
www.sccollege.eduTrigonal Pyramidal (AX 3 E) 2 2 Bent (AX 2 E 2) 120 4 109.5 3 Trigonal Planar (sp 2) Tetrahedral (sp 3) Geometry Examples VSEPR Predicting Molecular Geometry and Hybridization . Electron Groups Bonding Groups Lone Pairs Electron Geometry (Hybridization) Molecular Geometry (VSEPR class) Approximate Bond Angles ...
The Shapes of Molecules - University of Sydney
www.sydney.edu.auSquare pyramidal Square planar SF6, SiF6 2–, AsF 6 – IF5, SF5 –, SbF 5 2– ICl4 –, XeF 4 Note: the electron pair distribution and the molecular geometry are different if there are lone pairs of electrons present. Having determined the arrangement of electron pairs, take off one “arm” of the structure for every lone pair present.