Search results with tag "Carbonyl"
12.112.112.1 Nomenclature and Structure of Carbonyl Group
ncert.nic.inCarbonyl Group π Fig.12.1 Orbital diagram for the formation of carbonyl group The carbon-oxygen double bond is polarised due to higher electronegativity of oxygen relative to carbon. Hence, the carbonyl carbon is an electrophilic (Lewis acid), and carbonyl oxygen, a nucleophilic (Lewis base) centre. Carbonyl
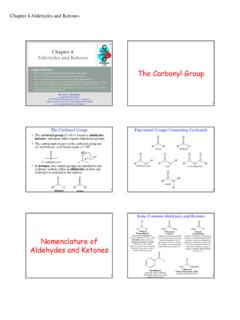
The Carbonyl Group - Angelo State University
www.angelo.eduThe Carbonyl Group 2 The Carbonyl Group • The carbonyl group (C=O) is found in aldehydes, ketones, and many other organic functional groups. • The carbon and oxygen in the carbonyl group are sp2-hybridized, with bond angles of 120°. •In ketones, two carbon groups are attached to the carbonyl carbon, while in aldehydes at least one
Chapter 23. Carbonyl Condensation Reactions
as.vanderbilt.eduChapter 23. Carbonyl Condensation Reactions As a result of the large dipole of the carbonyl group: 1. The carbonyl carbon is electrophilic and is the site of addition reactions by nucleophiles; 2. The α-protons are acidic and can be deprotonated by strong bases to give an enolate, which are nucleophiles and react with electrophiles. C CH O B ...
Ketones and Aldehydes - Rutgers University
crab.rutgers.eduThe carbonyl group is of central importance in organic chemistry because of its ubiquity. Without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The simplest carbonyl compounds are aldehydes and ketones.
Functional Groups - Purdue University
www.chem.purdue.edu• contain a C=O (“carbonyl”) group • note that in condensed structural formulas, the aldehyde group may be written as –CH=O or as –CHO I. Carboxylic Acids • contain a “carboxylic acid” group – a carbonyl (C=O) group bonded to a hydroxyl group at the carbonyl carbon atom
POLAR OR NON-POLAR PROPERTIES OF SOME …
www.columbia.edu2. Carbonyl is a general term for anything with the formula -- -- whether aldehyde or ketone. 3. A carboxyl does not have the same properties as a carbonyl plus a hydroxyl. The H in a carboxyl usually ionizes and the H in a hydroxyl usually does not. In other words, a carboxyl is not considered equivalent to a hydroxyl plus a
19.3 SPECTROSCOPY OF ALDEHYDES AND KETONES
www.saplinglearning.com900 CHAPTER 19 • THE CHEMISTRY OF ALDEHYDES AND KETONES. CARBONYL-ADDITION REACTIONS Like conjugated dienes, the p electrons of compounds in which carbonyl groups are con-jugated with double or triple bonds have strong absorption in the UV spectrum.
O O E+ E - University of Texas at Dallas
www.utdallas.eduEnols and Enolates! A type of reaction with carbonyl compounds is an α-substitution! (an electrophile adds to the α carbon of a carbonyl)! O O
硫化カルボニル Carbonyl sulfide COS - komyokk.co.jp
www.komyokk.co.jp179 硫化カルボニル Carbonyl sulfide COS 1.別 名 酸化硫化水素 オキシ硫化炭素 4.他の分析方法 ガスクロマトグラフ法[NIOSH]
TYPES OF CARBONYL COMPOUNDS - Chemistry2011.org
www.chemistry2011.orgTYPES OF CARBONYL COMPOUNDS General Name Name formula suffix O -al Aldehyde C R H O Ketone C -one R R' O -oic C acid Carboxylic acid H R O Acid O X=halogen -oyl halide C halide R X
PRODUCT NAME: CARBONYL SULFIDE 1. Chemical Product …
www.chinesedrywall.comMATERIAL SAFETY DATA SHEET PRODUCT NAME: CARBONYL SULFIDE MSDS: G-21 Revised: 6/7/96 Page 1 of 6 1. Chemical Product and Company Identification
O NUC O H C CH H C CH NUC O - The University of Texas at ...
utdallas.eduReactions at α-Position! In preceding chapters on carbonyl chemistry, a common reaction mechanism observed ! was a nucleophile reacting at the electrophilic carbonyl carbon site!
Q - knockhardy.org.uk
www.knockhardy.org.ukCARBONYL COMPOUNDS - Aldehydes and Ketones Structure • carbonyl groups consists of a carbon-oxygen double bond • the bond is polar due to the difference in electronegativity • aldehydes / ketones differ in what is attached to the carbon
Organic Chemistry | Topic Notes
studyclix.blob.core.windows.netcarbonyl group for aldehydes is sometimes written as C=OH or CHO as it’s at the end of a chain there is a hydrogen as well. The ending for aldehydes is -anal (eg: propanal) and for ketones it’s -anone (eg: propanone). The result of the addition of the carbonyl group is the boiling points and solubility change. Boiling Point
Transition Metal Carbonyls
alpha.chem.umb.educarbonyl product, which is greater for a low-valentmetal. Note that the first step in the case of an aldehydeis oxidative addition of the aldehydeC−H bond. It is much more difficult for the metal to break into a C−C bond so ketones, R 2CO, are usually resistant to this reaction.
Chapter 17: Alcohols and Phenols
as.vanderbilt.edu17.5: Alcohols from reduction of carbonyl compounds add the equivalent of H 2 across the π-bond of the carbonyl to give an alcohol RR' C O[H] R' RH H aldehyde (R or R´= H) → 1° alcohol ketone (R and R´≠ H) → 2° alcohol [H]: sodium borohydride: NaBH 4, ethanol reduces aldehydes to 1° alcohols and ketones to 2° alcohols
Fundamentals of Organic Chemistry 7 Carbohydrates
www.chtf.stuba.skthe carbonyl group: if the hydroxy group points to right when the carbonyl is “up” it is the D-isomer, and when the hydroxy group points to the left, it is the L-isomer. CHO HO H OHH CHO CH2OH HO H CH2OH H OH L-erythrose D-erythrose
CARBANIONS - SIUE
www.siue.eduaddition at the carbonyl group of another molecule. This process is a very important synthetic procedure and is known as the Aldol Condensation. The final product from aliphatic aldehydes or ketones contains both a carbonyl and an alcohol group. The product is called an aldol. CH 3CH=O CH 2CH=O CH 2=CH-O OH CH 3CH=O CH 2CH=O CH 3CH-O CH 2CH=O H ...
AMIDES AND RELATED FUNCTIONAL GROUPS
webhome.auburn.edunitrogen substituent is a carbonyl moiety. This structural modification produces a significant change in physicochemical properties of amides versus amines. Most importantly, amides are characterized by a "conjugated system" in which the NBEs of nitrogen can delocalized into the adjacent carbonyl (C=O) group.
Chapter 3: Organic Compounds: Alkanes and Cycloalkanes
www.vanderbilt.eduCarbonyl-oxygen double bonds (carbonyls) aldehyde ketones CO O H CO O C carboxylic acid ester CCl O acid chloride CO O C O anhydrides CN O amide acidic. 3 Alkanes and Alkane Isomers Alkanes: organic compounds with only C-C and C-H single (s) bonds. general formula for alkanes: CnH(2n+2) Saturated hydrocarbons Hydrocarbons: contains only carbon ...
NCERT
ncert.nic.inof carbonyl compounds on the basis of difference in their reactivity. Following tests are performed for the identification of aldehydic and ketonic groups: (i) On reaction with 2,4-dinitrophenylhydrazine (2,4-DNP), they form the respective 2,4–dinitrophenyl hydrazones. 24 …
926 CHAPTER 19 • THE CHEMISTRY OF ALDEHYDES AND …
www.saplinglearning.com926 CHAPTER 19 • THE CHEMISTRY OF ALDEHYDES AND KETONES. CARBONYL-ADDITION REACTIONS Notice in this synthesis that all steps following acetal formation involve basic or neutral condi-tions. Acid can be used only when destruction of the acetal is desired.
Overview of Carbonyl Compounds. CO. No leaving group ...
www.chem.uky.eduApache/2.4.7 (Ubuntu) Server at www.chem.uky.edu Port 80
REVERSIBLE ADDITION REACTIONS OF ALDEHYDES AND …
www.saplinglearning.com908 CHAPTER 19 • THE CHEMISTRY OF ALDEHYDES AND KETONES. CARBONYL-ADDITION REACTIONS To complete the nucleophilic addition, the negatively charged oxygen—an alkoxide ion, and a relatively strong base—is protonated by either water or HCN.
Beginners Guide to Soxhlet Extractions - Erowid
www.erowid.orgcompounds or impurities. This method of separation relies on the solubility characteristics of the particular species involved. ... As an alternative one could use acetone but the carbonyl with heat may lend to reaction with the specie of interest. SUMMARY
lecture 7 (stu - University of Birmingham
chemweb.bham.ac.ukNote that under acidic conditions imines can be activated in a similar way to aldehydes. Furthermore since the imine nitrogen is more basic than the oxygen in a carbonyl …
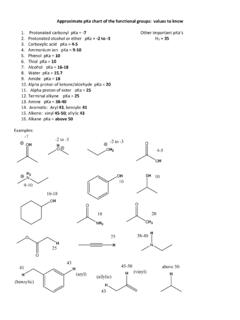
functional group pka - Department of Chemistry
www.chem.indiana.eduApproximate pKa chart of the functional groups: values to know 1. Protonated carbonyl pKa = ‐7 Other important pKa’s 2. Protonated alcohol or ether pKa = ‐2 to ‐3 H2 = 35 3.
Reactions of Amines
web.mnstate.eduFrom Aldehydes or Ketones: Reductive Amination (Section 19-19) R R 1 O Ketone or aldehyde + H N R 3 R 2 NaBH 3C cat.H+ R R H 1 N 2R via N • Access: 1º, 2º, or 3º Amines • Mechanism: Not required. (Basic workup) • The carbonyl reactant can be an aldehyde or a ketone • The amine reactant must have at least one hydrogen, as shown above ...
Fundamentals of Biochemistry - AgriMoon
agrimoon.comBiochemistry, as the name implies, is the chemistry of living organisms. Living organisms, whether they are microorganisms, plants or animals are basically ... Fundamentals of Biochemistry. ... The simplest monosaccharide that possesses a hydroxyl group and a carbonyl group with an asymmetric carbon atom is the aldotriose -glyceraldehyde. (A ...
SAMPLE LAB REPORT - Pittsburg State University
www.pittstate.eduFree radical compounds are very reactive and are involved in reactions such as hydrogen abstraction, radical coupling, and polymerizations. The second step of this reaction series is the acid catalyzed dehydration of benzopinacol ... absorbancies at 3050 and 1680 cm-1 for sp3 C-H and carbonyl stretches, respectively. The benzopinacol FT-IR ...
Organometallic Chemistry - uni-regensburg.de
www-oc.chemie.uni-regensburg.deketones, alkyl halides, and esters. – Relative reactivity: RCOCl > RCHO > tosylates, iodides > epoxides > bromides >> ketones > esters > nitriles. • In reactions with α,β-unsaturated carbonyl compounds, the organocopper reagents prefer 1,4-addition …
Overview of Carbonyl Compounds. CO. No leaving ... - Chemistry
www.chem.uky.eduApache/2.4.7 (Ubuntu) Server at www.chem.uky.edu Port 80
13 UnitUnitUnit - NCERT
ncert.nic.inalkyl or aryl group takes place from carbonyl carbon of the amide to the nitrogen atom. The amine so formed contains one carbon less than that present in the amide. Write chemical equations for the following conversions: (i) CH 3 –CH 2 –Cl into CH 3 –CH 2 –CH 2 –NH 2 (ii) C 6 H 5 –CH 2 –Cl into C 6 H 5 –CH 2 –CH 2 –NH 2 ...
19.10 ACETALS AND THEIR USE AS PROTECTING GROUPS
www.saplinglearning.com922 CHAPTER 19 • THE CHEMISTRY OF ALDEHYDES AND KETONES. CARBONYL-ADDITION REACTIONS The formation of acetals is reversible. The reaction is driven to the right either by the use of excess alcohol as the solvent or by removal of the water by-product, or both.
Carbonyl Chemistry - Fundamentals
www.chem.ucla.eduCarbonyl Chemistry - Fundamentals Images and Information from: Bruice, P. Organic Chemistry, Pearsons Prentice Hall. 2004 Hardinger, S. Chemistry 14D: Thinkbook. 2006.Lecture Supplement: Carbonyl Chemistry Fundamentals Carbonyl group – a carbon double bonded to an oxygen Acyl group – carbonyl group attached to an alkyl or aryl group Carbonyl compounds- compounds containing carbonyl …
Carbonyl Chemistry (12 Lectures)
www.ch.ic.ac.ukCarbonyl Group Reactions ¥Addition Reactions ÐCarbonyl groups in aldehydes and ketones undergo addition reactions. ÐThis is one of the most important reactions of the carbonyl group. O C + O C E E Y Y ¥Addition reactions occur by two different mechanisms: ÐBase-catalyzed addition (under basic or neutral conditions)
Carbonyl Chemistry: Survey of Reactions and Mechanisms
www.chem.ucla.eduCarbonyl Chemistry: Survey of Reactions and Mechanisms: When dealing with Carbonyls, we consider two general mechanism types: Carbonyls Have X as a Leaving Group Don’t have X as a Leaving Group
Carbonyl Chemistry – Fundamentals - UCLA
www.chem.ucla.eduCarbonyl Chemistry – Fundamentals Introduction Carbonyl group: Carbonyl functional groups: Brief Nomenclature of Aldehydes & Ketones1 IUPAC system:
carbonyl fundamentals - UCLA
www.chem.ucla.eduo Nucleophilic Carbonyl Substitution: LG is present. Occurs with ester, amide, anhydride, and acid chloride functional groups. Accept electrophile (usually H+) at oxygen
Similar queries
Carbonyl group, Carbonyl, Angelo State University, Chapter 23. Carbonyl Condensation Reactions, Bases, Ketones and Aldehydes, Group, POLAR PROPERTIES OF SOME, 19.3 SPECTROSCOPY OF ALDEHYDES AND KETONES, Chemistry, University of Texas at Dallas, Carbonyl sulfide, Acid Carboxylic acid, Acid, Chemical Product and Company Identification, CARBONYL COMPOUNDS, Organic Chemistry, Addition, Ketones, Aldehydes, And ketones, Amides, Organic Compounds: Alkanes and Cycloalkanes, Compounds, Of carbonyl compounds, ALDEHYDES AND, ALDEHYDES AND KETONES. CARBONYL-ADDITION REACTIONS, Overview of Carbonyl Compounds. CO. No leaving, REVERSIBLE ADDITION REACTIONS OF ALDEHYDES, CARBONYL-ADDITION REACTIONS, Erowid, Lecture 7 stu, Functional group pka, Reactions, Fundamentals of Biochemistry, Pittsburg State University, Organometallic Chemistry, NCERT, AND THEIR USE AS PROTECTING GROUPS, Carbonyl Chemistry 12 Lectures, Carbonyl fundamentals, Amide