Search results with tag "62304"
Evidence Product Checklist For Standard IEC 62304:2006 ...
images.techstreet.comintroduction. The size of these documents could vary from paragraphs to volumes depending upon the size and complexity of the project or business requirements. IEC 62304:2006 Information technology – Medical device software – Software life ... The information was transferred into checklist tables, based on the type of product or evidence.
Which OS for IEC 62304 Medical Systems? A Comparison of ...
blackberry.qnx.comWhich OS for IEC 62304 Medical Systems? A Comparison of Required Development Effort with Linux and the QNX Neutrino ... Introduction . A recurring question in discussions around software for medical systems is ... proprietary OS can be grouped into two categories: OS characteristics and cost.
Formation ISO 14971 et IEC 62304- Gestion des risques pour ...
www.id2sante.frFormation ISO 14971 et IEC 62304- Gestion des risques pour les dispositifs médicaux logiciels . Formation inter -entreprises du 15 février 2018, 9h-17h . CCI Bretagne (Centre-ville de Rennes), 1 Rue du Général Maurice Guillaudot (salle chalotais)
INTERNATIONAL IEC STANDARD 62304
webstore.iec.ch62304 IEC:2006 – 13 – Figure 1 – Overview of software development . PROCESSES . and . ACTIVITIES. Figure 2 – Overview of software maintenance . PROCESSES. and . ACTIVITIES. This standard identifies two additional . PROCESSES. considered essential for developing safe . MEDICAL DEVICE SOFTWARE. They are the software configuration ...
Introduction into IEC 62304 Software life cycle for ...
www.spiq.com9/5/2008 1 Navigation Introduction into IEC 62304 Software life cycle for medical devices Christoph Gerber 4. September 2008 SPIQ
よくある質問 - meti.go.jp
www.meti.go.jp参考和訳 3 序文 faq 62304 の目的 国際規格iec 62304(「医療機器ソフトウェア-ソフトウェアライフサイクルプロセス」)は、医療用ソ
MISRA Compliance:2020
www.misra.org.ukIEC 61508 [6], ISO 26262 [7], EN 50128 [8], IEC 62304 [9] and DO-178 [10]. 2.3 Training In order to ensure an appropriate level of skill and competence on the part of those who produce the source code, formal training should be provided for: The ...
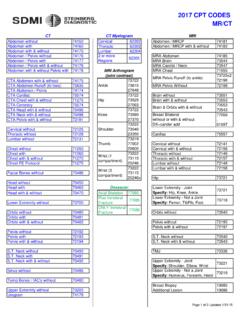
CPT Codes 2017
sdmi-lv.comAbdomen without 74150 Cervical 62302 Abdomen / MRCP 74181 Abdomen with 74160 Thoracic 62303 Abdomen / MRCP with & without 74183 Abdomen with & without 74170 Lumbar 62304
34260 3労 3 - mhlw.go.jp
www.mhlw.go.jpx 様式第16号の5(1)(裏面) イ.住居から就業の場所への移動 ロ.就業の場所から住居への移動 (リ)災害時の通勤の種別 ハ.就業の場所から他の就業の場所への移動 (該当する記号を記入) ニ.イに先行する住居間の移動 ホ.ロに後続する住居間の移動
34260 3労 1 - mhlw.go.jp
www.mhlw.go.jpx 様式第7号(1)(裏面) (リ) (ヌ) (ル) 労働者の 負傷又は発病の時刻 職名 前後 災害発生の 所属事業場の 事実を確認 名称・所在地 午 時 分頃 氏名
HIGH SPEED Dual Indication Fuse-Links HSDI
www1.cooperbussmann.comHIGH SPEED Dual Indication Fuse-Links HSDI Square Body DIN 43620 - 690V (IEC/UL), Size 000 to 00, 10 to 315 Amps Form No. HSDI 000/00 Page 1 of 5
IHLP Commercial Inductors, Low DCR Series - Vishay
www.vishay.comIHLP-2020CZ-11 www.vishay.com Vishay Dale Revision: 12-Oct-17 1 Document Number: 34260 For technical questions, contact: magnetics@vishay.com THIS DOCUMENT IS SUBJECT TO CHANGE WITHOUT NOTICE.