Search results with tag "Medwatch"
FDA Home Page | About MedWatch | Contact …
www.toledolaw.comFDA Logo--links to FDA home ... Department of Health and FDA Home Page | About MedWatch | Contact MedWatch | MedWatch Partners ... Intramuscular Use Only
for MANDATORY reporting MEDWATCH - QI equip
www.qiequip.comFDA USE ONLY H. DEVICE MANUFACTURERS ONLY Department of Health and Human Services Food and Drug Administration - MedWatch ... FORM FDA 3500A (10/05) (continued) MEDWATCH
www.fda.gov/medwatch DRUG INTERACTIONS FOR …
hemonc.orgCompany at 1-800-264-4662 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch ... is intended for use with aspirin and has been studied only in patients ...
A MEDWATCH CONTINUING EDUCATION ARTICLE
biotech.law.lsu.edul i c e n s u r e wh i c h may only occur after wide-scale use of the vaccine in the gener - ... (FDA), Rockville, MD ... MEDWATCH Continuing Education ...
COVID-19 Monoclonal Antibody (mAb)Therapeutics Provider ...
www.dshs.texas.gov• Mail to MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787, or • Fax (1-800-FDA-0178), or • Call 1-800-FDA-1088 to request a reporting form. • In addition, please provide a copy of all FDA MedWatch forms to: Regeneron Pharmaceuticals, Inc . Fax: 1-888-876-2736 . E-mail: : medical.information@regeneron.com
www.fda. gov/medwatch. see Dose Adjustment
www.fffenterprises.comPharmacovigilance at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda. gov/medwatch. ... • The Hizentra vial is for single-use only.
Analysis of Time to FDA Medwatch Safety Alert for ...
www.phusewiki.orgAnalysis of Time to FDA Medwatch Safety Alert for Therapeutic Monoclonal Antibodies ... only data on mAbs for which the alerts have been already
EDWATCH FDA USE ONLY Page of - St. John's …
facpub.stjohns.eduFDA USE ONLY Form Approved: ... www.fda.gov/medwatch/report.htm -- To report online ... Use section D for all products except medical devices
FDA’s Bad Ad Program - nahnnet.org
nahnnet.orgFDA’s Bad Ad Program ... 2.A way to get safety information OUT from FDA www.fda.gov/medwatch Who should report? ... risk…Sometimes there are risks that only …
For use by user-facilities, importers, distributors and ...
users.wpi.eduFDA USE ONLY H. DEVICE MANUFACTURERS ONLY Department of Health and Human Services ... www.fda.gov/medwatch/report.htm -- To report online NO POSTAGE NECESSARY IF MAILED
ADVERSE EVENT REPORTING – AN FDA …
www.aaos.orgADVERSE EVENT REPORTING – AN FDA REQUIREMENT WHAT, WHEN, ... the FDA introduced MedWatch, ... FDA USE ONLY Form Approved: ...
What Attorneys Should Know About FDA’s …
www.analysisgroup.comWhat Attorneys Should Know About FDA’s ... reports in the FDA’s MedWatch ... drugs cannot legally be sold without the FDA’s approval, which is awarded only ...
DOSAGE AND ADMINISTRATION ... - Bausch & Lomb
www.bausch.comBesivance is for topical ophthalmic use only, and should not be injected subconjunctivally, nor should it be ... at 1-800-FDA-1088 or www.fda.gov/medwatch.
A CONTINUING EDUCATION ARTICLE MEDWATCH
www.caliso9000.comto remain in general use, but are subject to an FDA request for safety and effective- ... are only as safe as the information known at that moment in time.
FDA Regulation of POC Blood Glucose Meters
www.whitehatcom.comFDA Regulation of POC Blood Glucose Meters ... - system should only be used with single-use, ... http://www.fda.gov/Safety/MedWatch/HowToReport/ucm0530
To report SUSPECTED ADVERSE REACTIONS, …
asclera.comFor intravenous use only. The strength of the solution and the volume ... North America, Inc. at 1-844-469-6379 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Baricitinib in COVID -19 Patients – Use Criteria and ...
www.amitahealth.orgmandatory reporting of all serious medication errors and adverse events considered potentially related to baricitinib-- Completion of : FDA MedWatch form is mandatory and onset . In hospitalized patients with COVID - 19, prophylaxis for VTE is recommended unless contraindicated. Pregnancy: Baricitinib should be used during pregnancy only if the
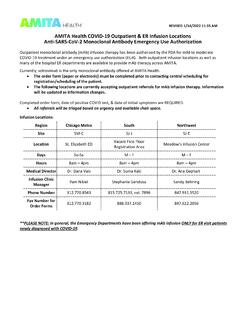
AMITA Health COVID-19 Outpatient & ER Infusion Locations ...
www.amitahealth.orgcommunicated to the local Pharmacy Department for evaluation and mandatory reporting to . FDA MedWatch. (This must be completed with 7 calendar days as stated per the EUA) **For additional information on sotrovimab, please refer to the . Fact Sheet for Healthcare Providers and available information on the
Frequently Asked Questions (FAQs)
www.tirfremsaccess.comFrequently Asked Questions (FAQs) 2 ... use only in opioid-tolerant patients. 2. ... You also may report adverse event information to the FDA MedWatch Reporting System by
Remdesivir Emergency Use Authorization (EUA) …
www.va.govJun 18, 2020 · Monitoring and Reporting of Adverse Events . ... o The prescribing provider is responsible for MANDATORY reporting of all serious medication errors and adverse events that COULD BE potentially related to remdesivir (those resulting in death, a life-threatening event, hospitalization or prolonging or ... report (a separate FDA MedWatch report is ...
Chapter 10 - Serious Adverse Event (SAE)
www.crcourses.comMEDWATCH FORM FDA 3500A (6/10) For use by user-facilities, importers, distributors and manufacturerers for MANDATORY reporting A. PATIENT INFORMATION B. ADVERSE EVENT OR PRODUCT PROBLEM C. SUSPECT PRODUCT(S) D. SUSPECT MEDICAL DEVICE E. INITIAL REPORTER Mfr Report# UF/Importer Report# FDA Use Only 1. Name (Give labled …
URGENT: Medical Device Recall
www.philips.comFDA’s MedWatch Adverse Event Reporting program either online, or by regular mail or fax. This notice has been reported to the appropriate Regulatory Agencies. Philips regrets any inconveniences caused by this problem. Sincerely, Rodney Mell Head of Quality and Regulatory Philips Respironics - Sleep & Respiratory Care
An Introduction to Drug Safety Surveillance and the FDA ...
www.fda.govMedWatch for reporting postmarketing safety ... consumer) to a ... • Passive and voluntary reports . 17. 18. Adverse Drug Experience as Defined by Regulation (21 CFR 314.80)
PRACTICE & NURSING Incident Report: Writing
www.ebscohost.comhealthcare facility may be required to report it to TJC, MedWatch (i.e., a medical products reporting system that is part of the U.S. Food and Drug Administration), and/or the U.S. Pharmacopeia Medication Errors Reporting Program ›The following should be performed before completing an IR:
FDA-REQUIRED REMS SAFETY INFORMATION
www.sublocaderems.comThe FDA has determined that a Risk ... SUBLOCADE is only available through a ... SUBLOCADE to the FDA at 1-800-FDA-1088 or online at www.fda.gov/medwatch…
HIGHLIGHTS OF PRESCRIBING INFORMATION Rx …
www.mitigare.comAmericas, Inc. at 1-877-233-2001 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. ... Use only if the potential benefit justifies the potential risk to
The eMDR Challenge - FDAnews
www.fdanews.comThe eMDR Challenge . 2 ... where it will automatically “load” the MedWatch file into the FDA’s databases. 5 ... The FDA will only allow you to storing up to 10
EDWATCH FDA USE ONLY Page of - aacap.org
www.aacap.orgFDA USE ONLY Form Approved: ... www.fda.gov/medwatch/report.htm -- To report online ... Use section D for all products except medical devices
HIGHLIGHTS OF PRESCRIBING INFORMATION ...
pi.amgen.com1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENTCOUNSELING INFORMATION and FDA-approved Patient Labeling. ... AIMOVIG is for subcutaneous use only.
AORN Guidance Statement: Reuse of Single-Use …
www.ascquality.orgAORN Guidance Statement: Reuse of Single-Use Devices ... devices intended to be used only once. The FDA’s ... Under MEDWATCH, the FDA’s medical device
MedWatch - Instructions for MedWatch Form 3500
medicalmarijuana.procon.orgshould generally not be submitted to FDA MedWatch as voluntary reports. ... copy of FDA Form 3500, with only section D or section E filled in as appropriate.
MEDWATCH FDA USE ONLY Page of - …
www.patientsafetyasap.orgFDA USE ONLY Form Approved: OMB ... http://www.fda.gov/medwatch/report/consumer/instruct.htm ... General Instructions for Completing the MedWatch Form FDA 3500
MEDWATCH Consumer Voluntary Reporting …
www.bcidaho.com• We ask only for the name and contact information of ... www.fda.gov/medwatch/report.htm. ... MedWatch Consumer Voluntary Reporting
MEDWATCH - Premier Safety Institute
www.premiersafetyinstitute.orgFDA USE ONLY H. DEVICE MANUFACTURERS ONLY Department of Health and Human Services Food and Drug Administration MedWatch; HFD-410 5600 Fishers Lane Rockville, MD 20857
MEDWATCH for MANDATORY reporting - restoresight
restoresight.orgThe public reporting burden for this collection of information has been estimated to average 66 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection ... MEDWATCH - for Mandatory Reporting
Similar queries
MedWatch, About MedWatch | Contact MedWatch | MedWatch Partners, Use only, For MANDATORY reporting MEDWATCH, FDA USE ONLY, ONLY, Reporting, FDA MedWatch, FDA Medwatch Safety Alert for Therapeutic Monoclonal Antibodies, FDA’s Bad Ad Program, ADVERSE EVENT REPORTING – AN FDA, ADVERSE EVENT REPORTING – AN FDA REQUIREMENT, MedWatch, ... FDA USE ONLY, What Attorneys Should Know About FDA, Bausch & Lomb, FDA Regulation of POC Blood Glucose, Mandatory reporting, Mandatory, Frequently Asked Questions FAQs, Remdesivir Emergency Use Authorization (EUA), For MANDATORY reporting, Chapter 10 - Serious Adverse Event SAE, Philips, Consumer, Voluntary, The eMDR Challenge, AORN Guidance Statement: Reuse of Single, MEDWATCH FDA USE ONLY, MedWatch Consumer Voluntary Reporting, MEDWATCH for MANDATORY reporting, MEDWATCH - for Mandatory Reporting