Search results with tag "Preferred reporting items for systematic review"
The Systematic Review: An Overview
alliedhealth.ceconnection.comconducted systematic review. Reporting standards similar to those produced for primary research de-signs have been created for systematic reviews. The PRISMA statement, or Preferred Reporting Items for Systematic Reviews and Meta-Analyses, pro-vides a checklist for review authors on how to re-port a systematic review.10 Ultimately, the quality
PRISMA-P (Preferred Reporting Items for Systematic review ...
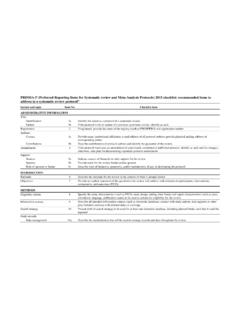
prisma-statement.orgPRISMA-P (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol* Section and topic Item No Checklist item ADMINISTRATIVE INFORMATION Title: Identification 1a Identify the report as a protocol of a systematic review Update 1b If the protocol is ...
AMSTAR 2: a critical appraisal tool for systematic reviews ...
www.bmj.comreviews, which led to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.3 The reporting guide for systematic reviews of observational (non-randomised) studies is MOOSE (Meta-analysis of Observational Studies in Epidemiology).4 The quality of reporting of a systematic review may, however, more accurately ...
The PRISMA 2020 statement: an updated guideline for ...
www.bmj.comThe Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have
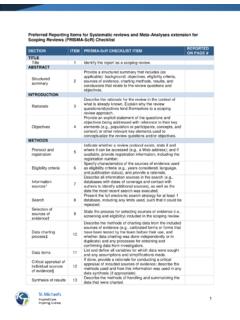
PRISMA 2009 checklist
www.prisma-statement.orgFunding 27 Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097.
The PRISMA 2020 statement: an updated guideline for ...
hbg.cochrane.orgThe Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement published in 2009 (hereafter referred to as PRISMA 2009) (4-7) is a reporting guideline designed to address poor reporting of systematic reviews (8). The PRISMA 2009 statement comprised a checklist
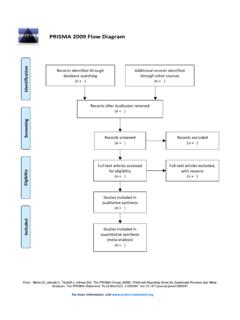
PRISMA 2009 flow diagram - PRISMA Statement
www.prisma-statement.orgFrom: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009).Preferred Reporting Items for Systematic Reviews and Meta- Analyses: The PRISMA Statement ...
Preferred reporting items for systematic review and meta ...
prisma-statement.orgchanges impact the results of a review. PRISMA-P: Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols A guideline to help authors prepare protocols for planned systematic reviews and meta-analyses that provides them with a minimum set of items to be included in the protocol. A protocol is intended to
Preferred Reporting Items for Systematic reviews and …
www.prisma-statement.orgto systematic reviews of interventions) to include and acknowledge the various sources of evidence that may be used in a scoping review (e.g., quantitative and/or qualitative research, expert opinion, and policy document). From: Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews
Systematic and Non-Systematic Literature Review ...
www.evidera.comMoher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Steward LA, and PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 Statement.