Search results with tag "Chemical kinetics"
Objectives Chemical Kinetics - NCERT
ncert.nic.inchemical kinetics. The word kinetics is derived from the Greek word ‘kinesis’ meaning movement. Thermodynamics tells only about the feasibility of a reaction whereas chemical kinetics tells about the rate of a reaction. For example, thermodynamic data indicate that diamond shall convert to graphite but
G a 12 C - Province of Manitoba
www.edu.gov.mb.caAppendix 3.3B: Chemical Kinetics: Assignment 2 (Answer Key) 13 Appendix 3.4A: Chemical Kinetics Problems 16 Appendix 3.4B: Chemical Kinetics Problems (Answer Key) 18 Appendix 3.5A: Factors Affecting the Rate of Reactions: Lab Activity 22 Appendix 3.5B: Factors Affecting the Rate of Reactions: Lab Activity (Answer Key) 26 Appendix 3.6A: Factors ...
Chapter 12 - Chemical Kinetics
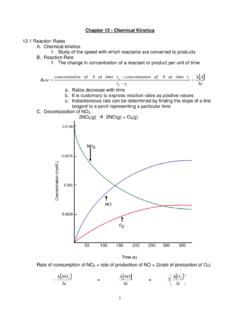
www.sciencegeek.net1 Chapter 12 - Chemical Kinetics . 12.1 Reaction Rates . A. Chemical kinetics 1. Study of the speed with which reactants are converted to products
Basic Principles and Calculations in Chemical Engineering
ceng.tu.edu.iqThese calculations with their applications in many chemical engineering fields ( mass transfer, heat transfer, chemical kinetics,…etc.) will be given in "Applied Mathematics in Chemical Engineering" within 3rd year of study. Chapter 7 A general Strategy for Solving Material Balance Problems The strategy outlined below is designed to focus ...
Syllabus of MAJOR ONLINE TEST SERIES TARGET Academic ...
s3-ap-southeast-1.amazonaws.comPeriodic properties, Basic inorganic nomenclature, Chemical thermodynamics & Thermochemistry GOC(complete) Chemical Bonding, Solid state, Chemical kinetics Isomerism, Chemical Equilibrium, State of matter (gaseous state),Redox & equivalent concept Ionic Equilibrium, Acid Base theory, Nuclear chemistry, Reaction intermediate.
Section Five Courses - catalog.caltech.edu
www.catalog.caltech.educourse will cover chemical equilibrium, chemical kinetics, combustion chemistry, transport phenomena, and the governing equations for multi-component gas mixtures. Topics will be chosen from non-premixed and ... Chemical Engineering.. -Engineering. -. . . . . - ...
Chapter 14. CHEMICAL EQUILIBRIUM
web.gccaz.eduChapter 14 Equilibrium Notes page 4 of 6 14.3 THE RELATIONSHIP BETWEEN CHEMICAL KINETICS AND CHEMICAL EQUILIBRIUM
Enzyme Kinetics - University Science Books - Home Page
www.uscibooks.comCHAPTER 10 Enzyme Kinetics One of the most fascinating areas of study in chemical kinetics is enzyme catalysis. The phenomenon of enzyme catalysis usually results in a very large increase in reac-
Chapter 14 Chemical Kinetics
alpha.chem.umb.eduChemical Kinetics Reaction Rates • A plot of concentration vs. time for this reaction yields a curve like this. • The slope of a line tangent to the curve at any point is the instantaneous rate at that time. C4H9Cl (aq ) + H 2O(l) → C4H9OH (aq ) + HCl (aq )
Fundamentals of Chemical Reactor Theory1
www.seas.ucla.eduChemical kinetics and reactor engineering are the scientific foundation for the analysis of most environmental engineering processes, both occurring in nature and invented by men. The need to quantify and compare processes led scientists and engineers throughout last century to develop what is now referred as Chemical Reaction Engineering (CRE).
Exercise 8 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE
www.fpharm.uniba.skChemical reactions, reaction rate Chemical kinetics is the part of physical chemistry that studies reaction rates. The reaction rate or rate of reaction for a reactant or product in a particular reaction is intuitively defined as how fast a reaction takes place. For example, the oxidation of iron under the atmosphere is
Copper electrowinning: theoretical and practical ... - SAIMM
www.saimm.co.zareaction kinetics. For the purposes of copper electrowinning reactor design it is necessary to determine the rate limiting step to optimize conditions so that capital costs and operating ability is optimized. Heterogeneous electron transfer By analogy with chemical kinetics for a simple first order reaction: [13] [14]
Reaction Kinetics: the Iodine Clock Reaction
www.fountainheadpress.comChemical kinetics is the study of reaction rate, or how fast a reaction proceeds. Knowing the factors that control the rate of reactions has tremendous implications in bothindustry and the environment. Manipulating these factors to increase the rate of a reaction can increase the yield
Mathematical Modelling in Systems Biology: An Introduction
www.math.uwaterloo.cachemical kinetics, providing rate laws for biochemical processes (i.e. enzyme-catalysed reactions and cooperative binding). An optional section treats common approximation methods. Chapter 4 introduces techniques for analysis of differential equation models, including phase plane analysis, stability, bifurcations, and sensitivity analysis.
CHAPTER 4:The Material Balance for Chemical Reactors
sites.engineering.ucsb.eduThis is the subject of chemical kinetics, Chapter 5 Here we use common reaction-rate expressions without derivation 4/152. The Batch Reactor R j The batch reactor is assumed well stirred Let the entire reactor contents be the reactor volume element 5/152
The Kinetics of the Iodine Clock Reaction
www.chemistrylabmanual.comThere is an entire spectrum of reaction speeds, ranging from very slow to extremely fast. For example, the rusting of iron is reasonably slow, whereas the decomposition of TNT is extremely fast. The branch of chemistry that is concerned with the rates of reactions is called chemical kinetics. Understanding reaction rates helps us control them and
CHEMISTRY (043) Class XI
cbseacademic.nic.inB. Chemical Kinetics a. Effect of concentration and temperature on the rate of reaction between Sodium Thiosulphate and Hydrochloric acid. b. Study of reaction rates of any one of the following: i) Reaction of Iodide ion with Hydrogen Peroxide at room temperature using different concentration of Iodideions.
Chemical Kinetics: A Laboratory Investigation of Rate Laws
www.uiltexas.orgChemistry Lesson to Prepare for UIL Science Contest Lesson Plan Title: Chemical Kinetics: A Laboratory Investigation of Rate Laws Goal of Lesson: To use actual laboratory data to determine a rate law, rate constant, and activation energy through application …
CHEMICAL KINETICS - knockhardy.org.uk
www.knockhardy.org.ukCHEMICAL KINETICS Reminder The following methods can be used to increase the rate of a reaction. • increase surface area • use a light source (certain reactions only)
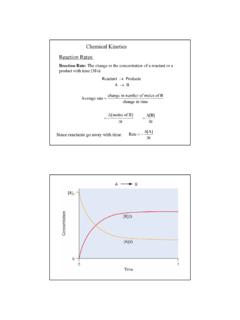
Chemical Kinetics Reaction Rates
www.csus.eduChemical Kinetics. 2 Consider the decomposition of N2O5 to give NO2 and O2: 2N2O5(g)→4NO2(g) + O2(g) reactants decrease with time products increase with time. 3 2N2O5(g)→4NO2(g) + O2(g) From the graph looking at t = 300 to 400 s 61 2 0.0009M Rate O = 9 10 Ms
Chemical Kinetics - NCERT
www.ncert.nic.indetermined from chemical equilibrium; (c) speed of a reaction i.e. time taken by a reaction to reach equilibrium. Along with feasibility and extent, it is equally important to know the rate and the factors controlling the rate of a chemical reaction for its complete understanding. For example, which parameters determine as to how rapidly food ...
Chemical Kinetics
users.cs.duke.eduOutline: Kinetics Reaction Rates How we measure rates. Rate Laws How the rate depends on amounts of reactants. Integrated Rate Laws How to calculate amount left or time to reach a given amount. Half-life How long it takes to react 50% of reactants. Arrhenius Equation How rate constant changes with temporature.
Similar queries
Chemical Kinetics, Kinetics, Reaction, Activity, Chapter 12 - Chemical Kinetics, Chemical Engineering, Chemical, Engineering, Chapter 14, CHEMICAL EQUILIBRIUM, Chapter 14 Equilibrium, Enzyme Kinetics, CHAPTER, Chemical Kinetics Reaction, Reaction Kinetics: the Iodine Clock Reaction, Of reaction, Balance for Chemical, CHEMISTRY, Rate, Parameters, Kinetics Reaction